Abstract
Purpose
To presents the effect of triple therapy including C3 F8 gas injection, intravitreal anti-VEGF injection and photodynamic therapy on patients with subretinal hemorrhage accompanied by choroidal neovascularization.
Methods
Twelve eyes of 12 patients suffering from subretinal hemorrhage accompanied by choroidal neovascularization with onset of the symptom within a week prior to three-day prone positioning after C3 F8 gas injection were included in the present study. Next, intravitreal anti-VEGF injection and photodynamic therapy was performed. Then, within two months, intravitreal bevacizumab or ranibizumab injection was performed.
Results
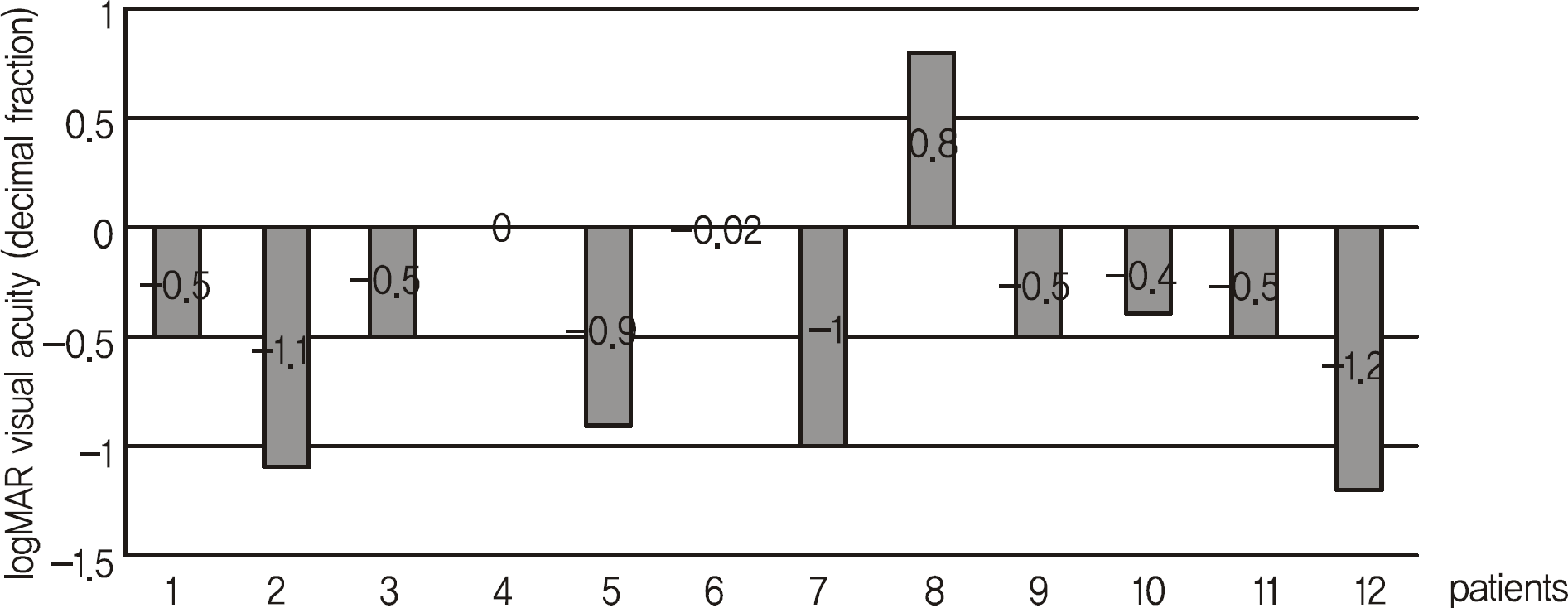
After stabilization of the submacular hemorrhagic lesion, ten eyes of ten patients showed improved visual acuity, one eye showed no improvement, and decreased visual acuity developed in one patient. LogMAR visual acuity improved after the initial treatment from 1.05 ± 0.43 to 0.74 ± 0.58 and 0.53 ± 0.51 at three and six months, respectively. The improvement was considered to be clinically significant.
Conclusions
An appropriate regimen for treating broad submacular hemorrhage accompanied by choroidal neovascularization has not been established. The authors of the patients had obtained positive results from C3 F8 gas injection, intravitreal anti-VEGF injection and photodynamic therapy. In the future, additional studies should be conducted.
Go to : 
References
1. Bennett SR, Folk JC, Blodi CF, Klugman M. Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol. 1990; 109:33–7.

2. Avery RL, Fekrat S, Hawkins BS, Bressler NM. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina. 1996; 16:183–9.

3. Scupola A, Coscas G, Soubrane G, Balestrazzi E. Natural history of macular subretinal hemorrhage in age-related macular degeneration. Ophthalmologica. 1999; 213:97–102.

4. Macular Photocoagulation Study Group. Subfoveal neovascular lesions in age-related macular degeneration. Guidelines for evaluation and treatment in the macular photocoagulation study. Arch Ophthalmol. 1991; 109:1242–57.
5. Emerson MV, Lauer AK, Flaxel CJ, et al. Intravitreal bevacizumab (Avastin) treatment of neovascular age-related macular degeneration. Retina. 2007; 27:439–44.

6. Bressler NM. Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-tap report 2. Arch Ophthalmol. 2001; 119:198–207.
7. Hesse L, Schmidt J, Kroll P. Management of acute submacular hemorrhage using recombinant tissue plasminogen activator and gas. Graefes Arch Clin Exp Ophthalmol. 1999; 237:273–7.

8. Meier P, Zeumer C, Jochmann C, Wiedemann P. Management of submacular hemorrhage by tissue plasminogen activator and SF (6) gas injection. Ophthalmologe. 1999; 96:643–7.
9. Hassan AS, Johnson MW, Schneiderman TE, et al. Management of submacular hemorrhage with intravitreous tissue plasminogen activator injection and pneumatic displacement. Ophthalmology. 1999; 106:1900–6. discussion 1906-7.
10. Bashshur ZF, Schakal A, Hamam RN, et al. Intravitreal bevacizumab vs verteporfin photodynamic therapy for neovascular age-related macular degeneration. Arch Ophthalmol. 2007; 125:1357–61.

11. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007; 143:566–83.

12. Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006; 26:859–70.

13. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–44.

14. Sanders D, Peyman GA, Fishman G, et al. The toxicity of intravitreal whole blood and hemoglobin. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1975; 197:255–67.

15. Stephen JR. Retina. 4th ed.1. Baltimore: Mosby;2005. p. 137–52.
16. Toth CA, Morse LS, Hjelmeland LM, Landers MB 3rd. Fibrin di-rects early retinal damage after experimental subretinal hemorrhage. Arch Ophthalmol. 1991; 109:723–9.

17. Bennett SR, Folk JC, Blodi CF, Klugman M. Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol. 1990; 109:33–7.

18. Berrocal MH, Lewis ML, Flynn HW Jr. Variations in the clinical course of submacular hemorrhage. Am J Ophthalmol. 1996; 122:486–93.

19. Hattenbach LO, Klais C, Koch FH, Gümbel HO. Intravitreous injection of tissue plasminogen activator and gas in the treatment of submacular hemorrhage under various conditions. Ophthalmology. 2001; 108:1485–92.

20. Johnson MW, Olsen KR, Hernandez E, et al. Retinal toxicity of recombinant tissue plasminogen activator in the rabbit. Arch Ophthalmol. 1990; 108:259–63.

21. Kamei M, Estafanous M, Lewis H. Tissue Tissue plasminogen activator in the treatment of vitreoretinal diseases. Semin Ophthalmol. 2000; 15:44–50.
Go to : 
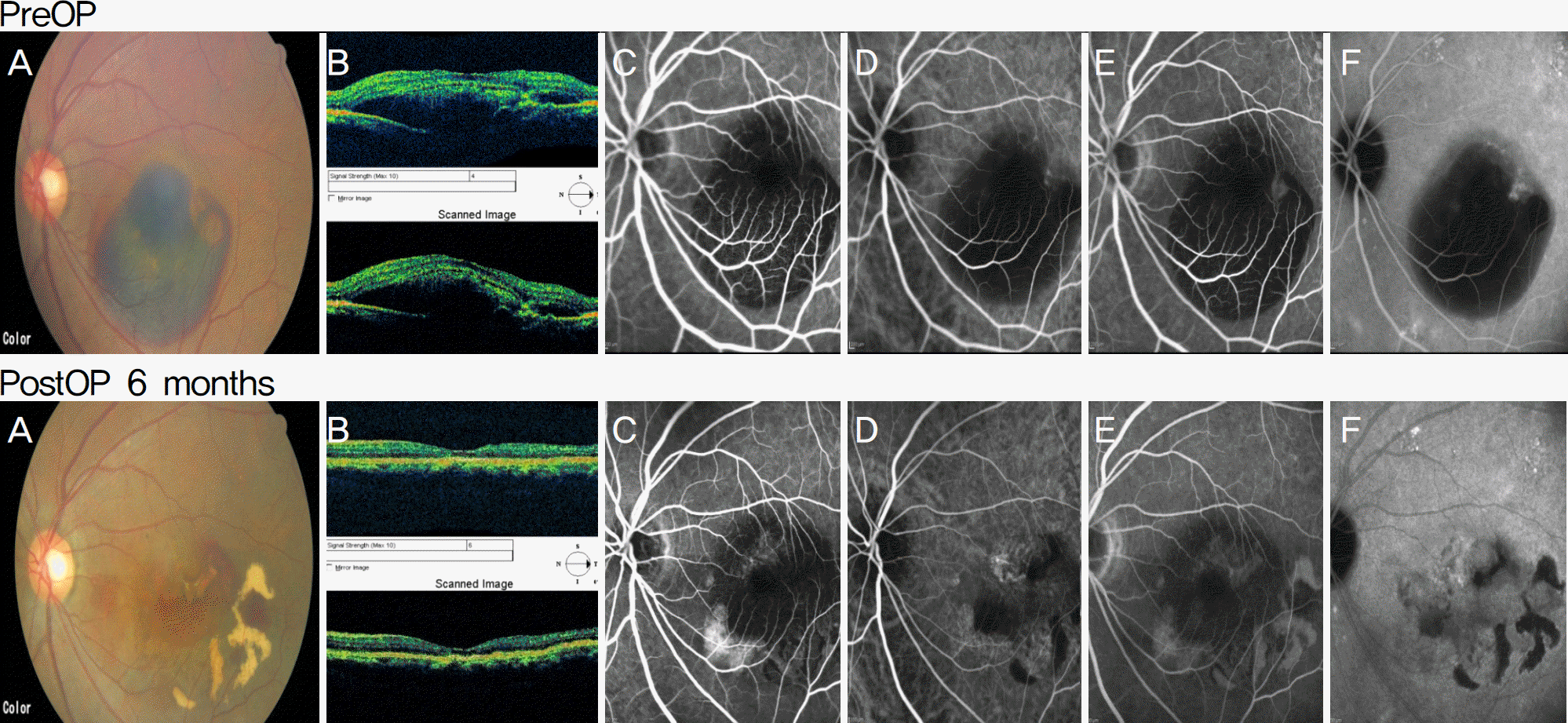 | Figure 1.Case 1 preoperation and post-operation fundus photograph, OCT, FAG and HRA. (A) Fundus photography, (B) OCT, (C) FAG (early phase), (D) HRA ICG (early phase), (E) FAG (late phase), (F) HRA ICG (late phase). |
Table 1.
Baseline demographics for patients
| case | Sex/age | Hemorrhage Diameter (DD) | BCVA§(initial) |
Systemic Disease |
Anti- VEGF | F/U (months) | |
|---|---|---|---|---|---|---|---|
| HTN | DM | ||||||
| 1 | F/47 | 3 | 20/80 | N† | Y‡ | Bevacizumab | 12 |
| 2 | M/79 | 3.5 | FC* | N | N | Bevacizumab | 13 |
| 3 | F/42 | 3.2 | FC | N | N | Bevacizumab | 7 |
| 4 | F/77 | 5.5 | FC | Y | N | Bevacizumab | 10 |
| 5 | M/79 | 3 | FC | Y | Y | Bevacizumab | 13 |
| 6 | M/67 | 3.1 | 20/50 | N | N | Bevacizumab | 8 |
| 7 | M/60 | 4 | 20/200 | N | N | Bevacizumab | 18 |
| 8 | M/67 | 3 | 20/100 | Y | N | Bevacizumab | 10 |
| 9 | M/74 | 4 | 10/200 | Y | N | Bevacizumab | 16 |
| 10 | F/49 | 3 | 20/100 | N | N | Ranibizumab | 9 |
| 11 | F/49 | 3 | 20/100 | N | N | Ranibizumab | 10 |
| 12 | F/62 | 3.3 | 10/200 | Y | Y | Bevacizumab | 11 |
Table 2.
Frequency distribution of changes in visual acuity from baselines
| Changes in visual acuity from baseline to month 6 | No. of treated eyes (n = 12) |
|---|---|
| V/A* improvement (n = 10) | |
| ≥ Six line increase | 1 (8.33%) |
| Four to < six line increase | 4 (33.33%) |
| Two to < four line increase | 5 (41.67%) |
| No change (< two line increase) | 1 (8.33%) |
| V/A* loss | 1 (8.33%) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download