Abstract
Purpose
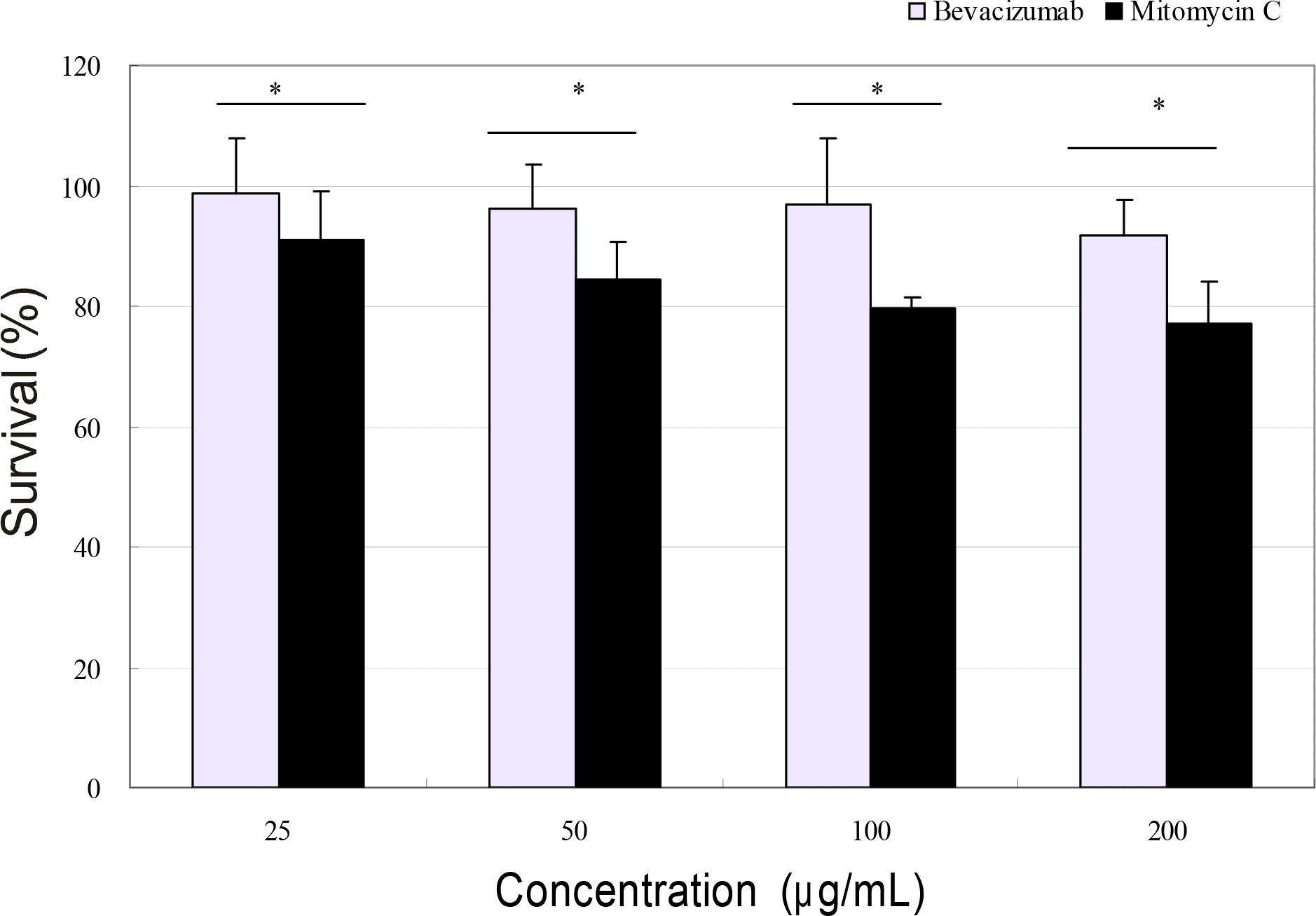
To compare the antiproliferative effects between bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF), and mitomycin C in human Tenon's capsule fibroblasts.
Methods
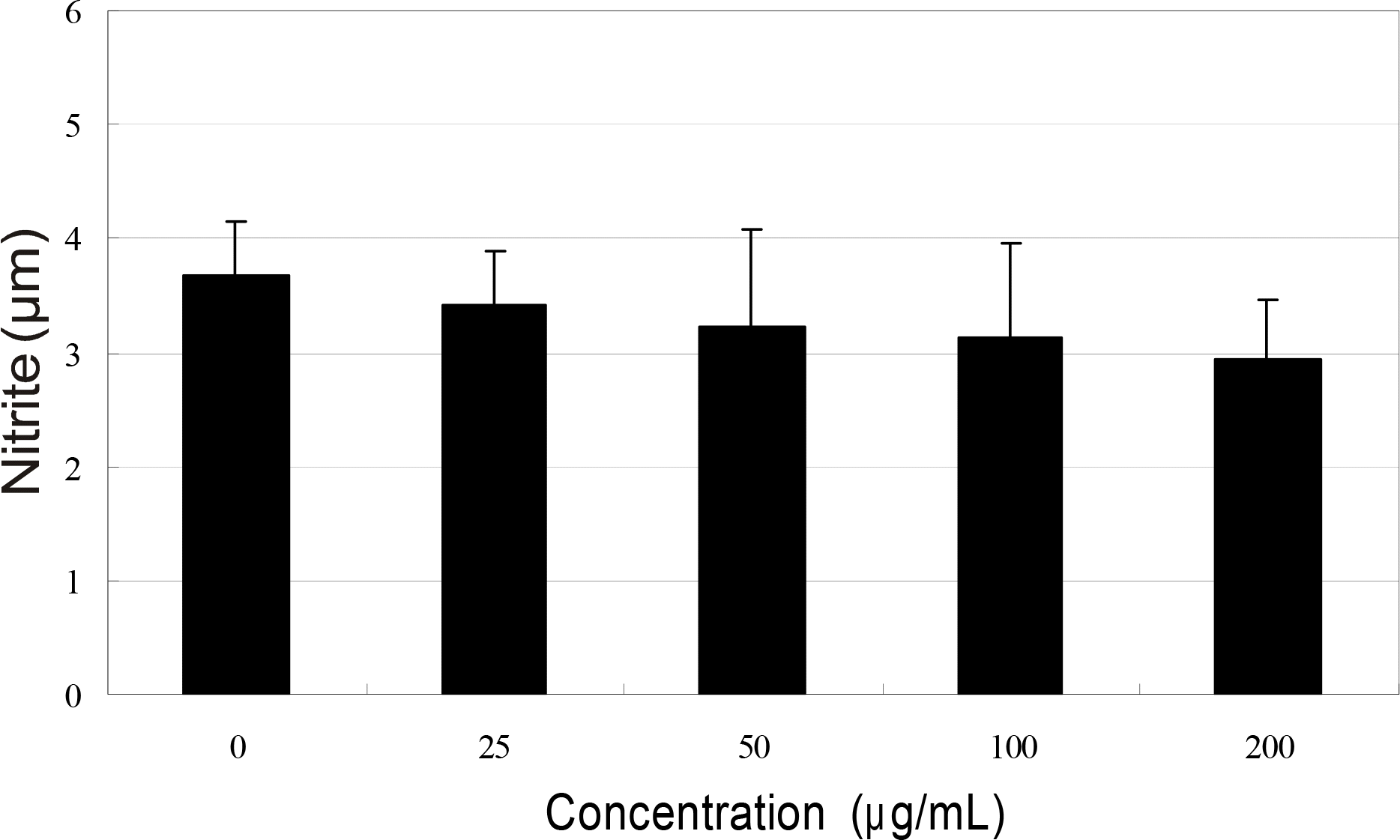
Primarily cultured human Tenon's capsule fibroblasts were exposed to 0, 25, 50, 100, and 200 μ g/ml bevacizumab and mitomycin C, and incubated for 5 days. Cellular survival and production of nitric oxide were assessed by MTT assay and Griess assay, respectively.
References
1. Midgley R, Kerr D. Bevacizumab–current status and future directions. Ann Oncol. 2005; 16:999–1004.

2. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006; 113:363–72.

3. Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006; 26:275–8.

4. Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina. 2006; 26:352–4.

5. Davidorf FH, Mouser JG, Derick RJ. Rapid improvement of ru-beosis iridis from a single bevacizumab (Avastin) injection. Retina. 2006; 26:354–6.

6. Skuta GL, Parrish RK 2nd. Wound healing in glaucoma filtering surgery. Surv Ophthalmol. 1987; 32:149–70.
7. Lama PJ, Fechtner RD. Antifibrotics and wound healing in glaucoma surgery. Surv Ophthalmol. 2003; 48:314–46.

8. Li Z, Van Bergen T, Van de Veire S, et al. Inhibition of vascular endothelial growth factor reduces scar formation after glaucoma fil-tration surgery. Invest Ophthalmol Vis Sci. 2009; 50:5217–25.

9. Bouloumié A, Schini-Kerth VB, Busse R. Vascular endothelial growth factor up-regulates nitric oxide synthase expression in endothelial cells. Cardiovasc Res. 1999; 41:773–80.

10. Morbidelli L, Chang CH, Douglas JG, et al. Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am J Physiol. 1996; 270:H411–5.

11. Crowston JG, Wang XY, Khaw PT, et al. Human serum reduces mitomycin-C cytotoxicity in human tenon's fibroblasts. Invest Ophthalmol Vis Sci. 2006; 47:946–52.

12. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.

13. Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982; 126:131–8.
14. Bouloumié A, Schini-Kerth VB, Busse R. Vascular endothelial growth factor up-regulates nitric oxide synthase expression in endothelial cells. Cardiovasc Res. 1999; 41:773–80.

15. Dulak J, Józkowicz A, Dembinska-Kiec A, et al. Nitric oxide induces the synthesis of vascular endothelial growth factor by rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000; 20:659–66.

16. Spitzer MS, Yoeruek E, Sierra A, et al. Comparative antiprolifer ative and cytotoxic profile of bevacizumab (Avastin), pegaptanib (Macugen) and ranibizumab (Lucentis) on different ocular cells. Graefes Arch Clin Exp Ophthalmol. 2007; 245:1837–42.
17. Iriyama A, Chen YN, Tamaki Y, Yanagi Y. Effect of anti-VEGF antibody on retinal ganglion cells in rats. Br J Ophthalmol. 2007; 91:1230–3.

18. Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology. 2007; 114:855–9.

19. Kernt M, Welge-Lüssen U, Yu A, et al. Bevacizumab is not toxic to human anterior- and posterior-segment cultured cells. Ophthalmologe. 2007; 104:965–71.
20. Kim SH, Kim JW. Effect of bevacizumab on survival and production of nitric oxide in trabecular meshwork cells. J Korean Ophthalmol Soc. 2009; 50:1404–8.

Figure 1.
Effect of bevacizumab on the survival of human Tenon's capsule fibroblasts. bevacizumab inhibited survival of fibroblasts at high concentration (∗ p<0.05).
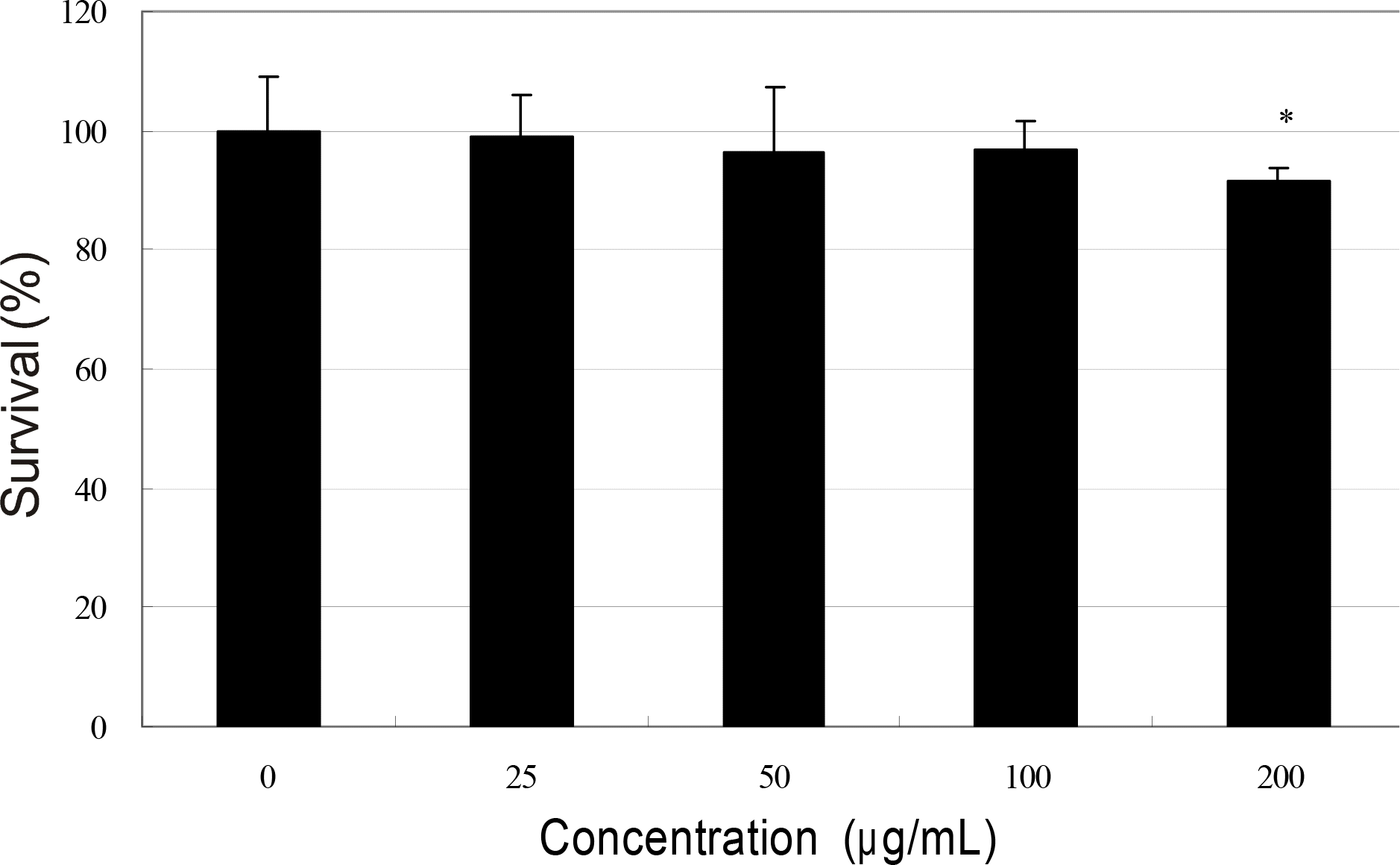
Figure 2.
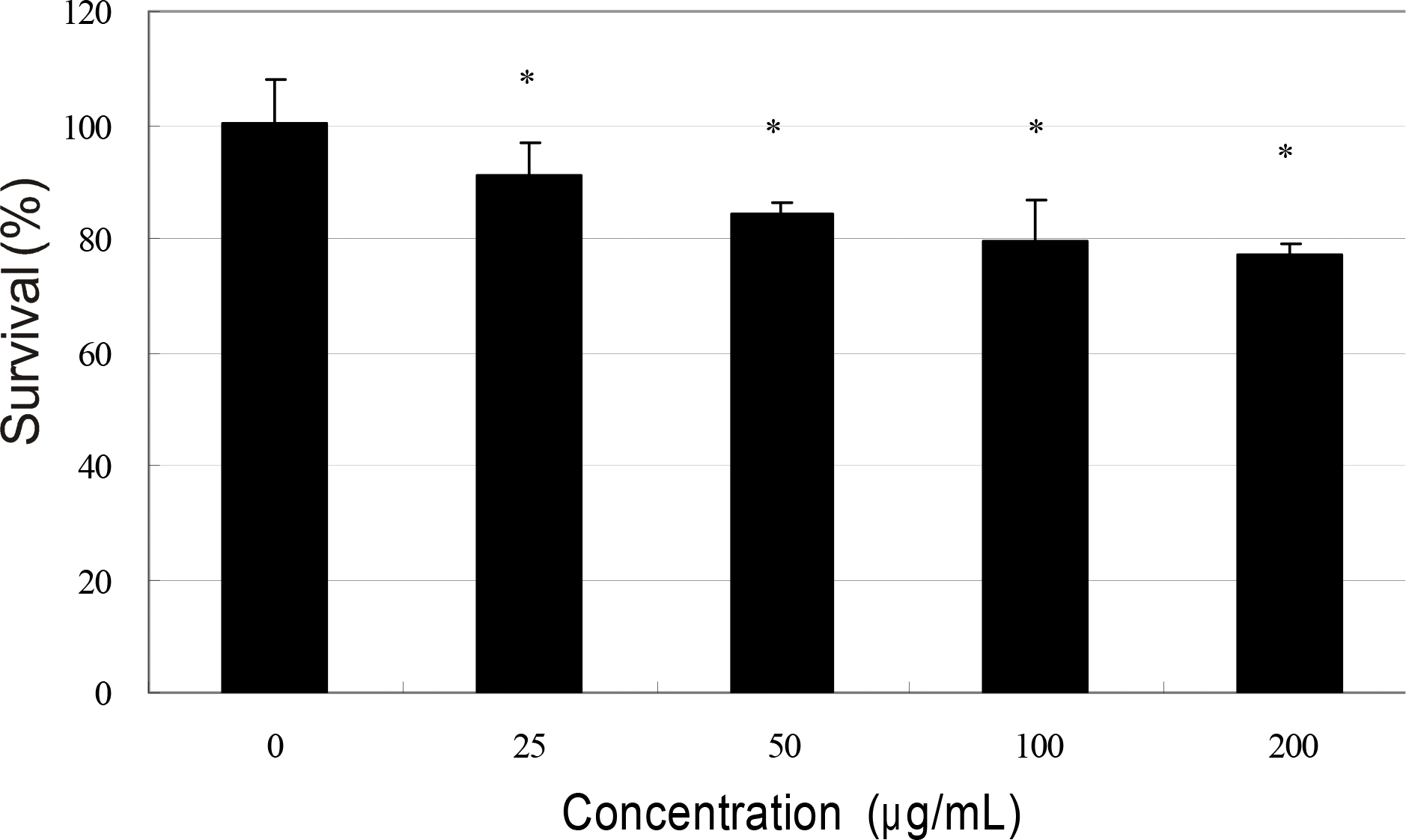
Effect of mitomycin C on the survival of human Tenon's capsule fibroblasts. Mitomycin C inhibited cellular survival significantly from low concentration (∗ p<0.05).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download