Abstract
Purpose
To evaluate the long-term therapeutic effects of intravitreal bevacizumab on myopic choroidal neovascularization (CNV).
Methods
Medical records of 6 patients who underwent intravitreal bevacizumab injection for myopic CNV and were followed for more than 2 years, were retrospectively investigated. The best corrected visual acuity was compared at 1,3,12, and 24 months after injection. Two years after the injection, a fluorescein angiography and optical coherence tomography (OCT) were performed to evaluate the central macular thickness and leakage of CNV.
Results
The mean best corrected visual acuity was 1.16 ± 0.43 (logMAR), 0.45 ± 0.21 (logMAR), 0.29 ± 0.23 (logMAR), 0.14 ± 0.11 (logMAR), and 0.11 ± 0.06 (logMAR) at baseline, 1, 3, 12, and 24 months after injection, respectively. The average number of injections was 1.33. In OCT, 2 years after bevacizumab injection, central foveal thickness was significantly decreased as compared to the baseline, and fluorescein angiography showed no leakage of fluorescein.
Go to : 
References
2. Curtin BJ. Physiologic vs pathologic myopia: genetic vs aberrations. Ophthalmology. 1979; 86:681–91.
4. Hayasaka S, Uchida M, Setogawa T. Subretinal hemorrhages with or without choroidal neovascularization in the maculas of patients with pathologic myopia. Graefes Arch Clin Exp Ophthalmol. 1990; 228:277–80.

5. Hotchkiss ML, Fine SL. Pathologic myopia and choroidal aberrations. Am J Ophthalmol. 1981; 91:177–83.
6. Levy JH, Pollock HM, Curtin BJ. The Fuchs' spot: an ophthalmoscopic and fluorescein angiographic study. Ann Ophthalmol. 1977; 9:1433–42.
7. Yoshida T, Ohno-Matsui K, Yasuzumi K, et al. Myopic choroidal neovascularization: a 10-year follow-up. Ophthalmology. 2003; 110:1297–305.
8. McCarty CA, Livingston PM, Taylor HR. Prevalence of myopia in adults: implications for refractive surgeons. J Refract Surg. 1997; 13:229–34.

9. Ohno-Matsui K, Yoshida T, Futagami S, et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularisation in pathological myopia. Br J Ophthalmol. 2003; 87:570–3.

10. Chan WM, Ohji M, Lai TY, et al. Choroidal neovascularisation in pathological myopia: an update in management. Br J Ophthalmol. 2005; 89:1522–8.

11. Secretan M, Kuhn D, Soubrane G, et al. Long-term visual outcome of choroidal neovascularization in pathologic myopia: natural history and laser. Eur J Ophthalmol. 1997; 7:307–16.
12. Steidl SM, Pruett RC. Macular complications associated with posterior staphyloma. Am J Ophthalmol. 1997; 123:181–7.

13. Ruiz-Moreno JM, Montero JA. Long-term visual acuity after argon green laser photocoagulation of juxtafoveal choroidal neovascularization in highly myopic eyes. Eur J Ophthalmol. 2002; 12:117–22.

14. Soubrane G. Choroidal neovascularization in pathologic myopia: recent developments in diagnosis and treatment. Surv Ophthalmol. 2008; 53:121–38.

15. Ruiz-Moreno JM, de la Vega C. Surgical removal of subfoveal choroidal neovascularisation in highly myopic patients. Br J Ophthalmol. 2001; 85:1041–3.

16. Verteporfin in Photodynamic Therapy (VIP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin 1-year results of a randomized clinical trial VIP report No. 1. Ophthalmology. 2001; 108:841–52.
17. Blinder KJ, Blumenkranz MS, Bressler NM, et al. aberrations therapy of subfoveal choroidal neovascularization in pathologic myopia 2-year results of a randomized clinical trial VIP Report No. 3. Ophthalmology. 2003; 110:667–73.
18. Chan WM, Lai TY, Kiu DT, Lam DS. Intravitreal bevacizumab (Avastin) for myopic choroidal neovascularization six-month results of a prospective pilot study. Ophthalmology. 2007; 114:2190–6.
19. Kim KH, Jung JH, Lee JE, Oum BS. Clinical effect of intravitreal bevacizumab injection in myopic choroidal neovascularization. J Korean Ophthalmol Soc. 2010; 51:359–65.

20. Gharbiya M, Allievi F, Mazzeo L, Gabrieli CB. Intravitreal bevacizumab treatment for choroidal neovascularization in pathologic myopia: 12-month results. Am J Ophthalmol. 2009; 147:84–93.

21. Ikuno Y, Sayanagi K, Soga K, et al. Intravitreal bevacizumab for choroidal neovascularization attributable to pathological myopia: one-year results. Am J Ophthalmol. 2009; 147:94–100.

22. Hampton GR, Kohen D, Bird AC. Visual prognosis of disciform degeneration in myopia. Ophthalmology. 1983; 90:923–6.

23. Hayashi K, Ohno-Matsui , Yoshida T. Characteristics of patients with a favorable natural course of myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2005; 243:13–9.

24. Avila MP, Weiter JJ, Jalkh AE, et al. Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology. 1984; 91:1573–81.

25. Fried M, Siebert A, Meyer-Schwickerath G. A natural history of Fuchs' spot: a long-term follow-up study. Doc Ophthalmol. 1981; 28:215–21.
26. Tabandeh H, Flynn HW Jr, Scott IU, et al. Visual acuity outcomes of patients 50 years of age and older with high myopia and untreated choroidal neovascularization. Ophthalmology. 1999; 106:2063–7.

27. Bottoni F, Perego E, Airaghi P, et al. Surgical removal of subfoveal choroidal neovascular membranes in high myopia. Graefes Arch Clin Exp Ophthalmol. 1999; 237:573–82.

28. Uemura A, Thomas MA. Subretinal surgery for choroidal neovascularization in patients with high myopia. Arch Ophthalmol. 2000; 118:344–50.

29. Yamamoto I, Rogers AH, Reichel E, et al. Intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularization secondary to pathologic myopia. Br J Ophthalmol. 2007; 91:157–60.
30. Hayashi K, Ohno-Matsui K, Teramukai S, et al. Comparison of visual outcome and regression pattern of myopic choroidal neovascularization after intravitreal bevacizumab or after photodynamic therapy. Am J Ophthalmol. 2009; 148:396–408.

31. Ruiz-Moreno JM, Montero JA. Intravitreal bevacizumab to treat myopic choroidal neovascularization: 2-year outcome. Graefes Arch Clin Exp Ophthalmol. 2010; 248:937–41.

32. Ikuno Y, Nagai Y, Matsuda S, et al. Two-year visual results for older asian women treated with photodynamic therapy or aberrations for myopic choroidal neovascularization. Am J Ophthalmol. 2010; 149:140–6.
33. Kakinoki M, Sawada O, Sawada T, et al. Comparison of macular thickness between cirrus HD-OCT and stratus OCT. Ophthalmic Surg Lasers Imaging. 2009; 40:135–40.

Go to : 
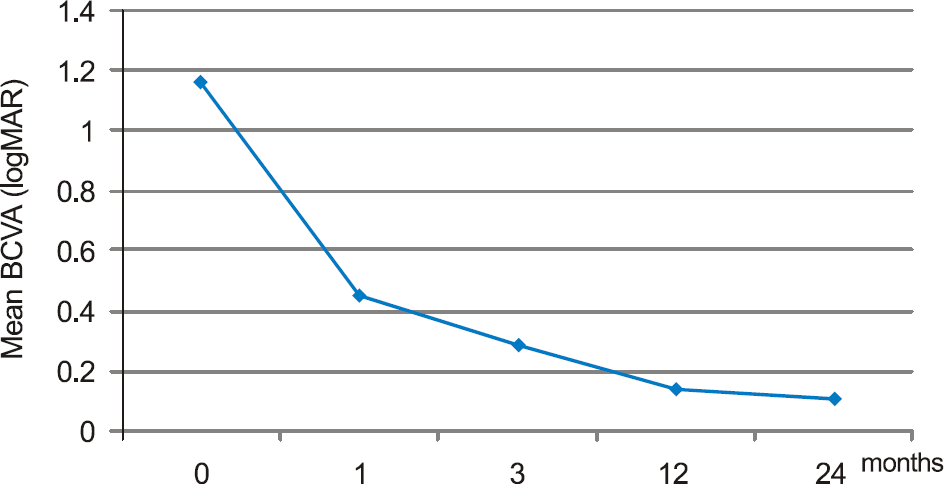 | Figure 1.Mean best corrected visual acuity at baseline, 1, 3, 12, and 24 months. After intravitreal bevacizumab injection, graph shows to maintenance of good visual acuity until 2 years later (p = 0.042, p = 0.028, p = 0.042, p = 0.028, Wilcoxon signed rank test). |
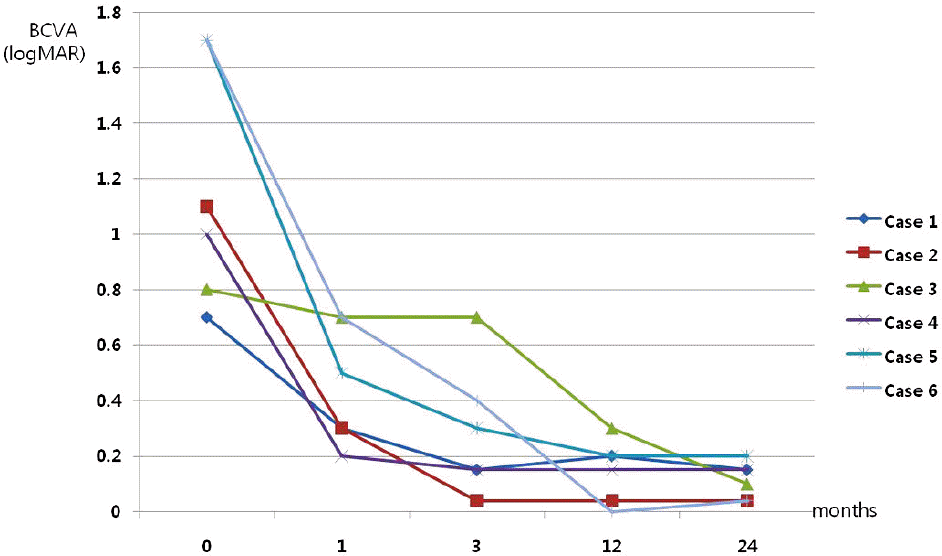 | Figure 2.Best corrected visual acuity in each of six cases. Most cases maintain improved visual acuity until 2 years later. |
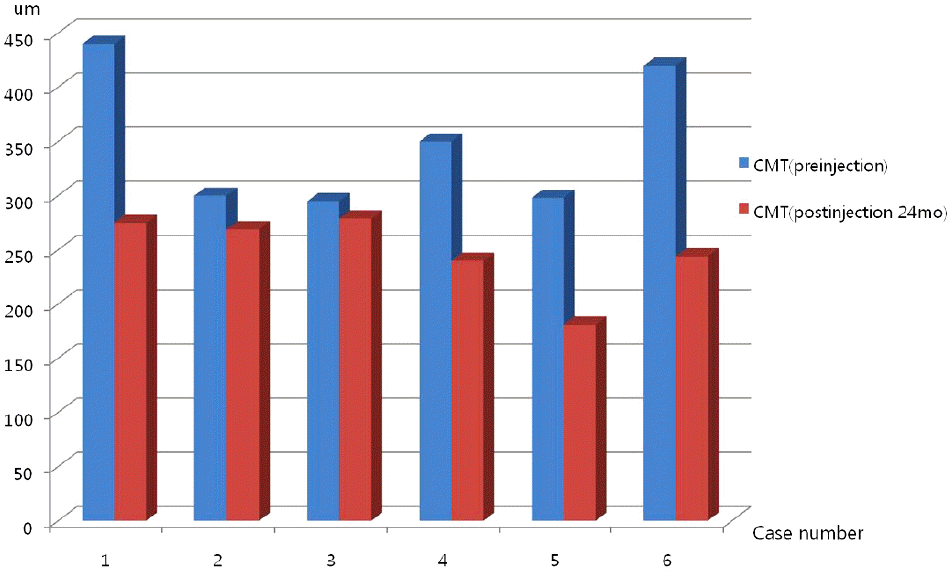 | Figure 3.CMT at preinjection and 2-years later after injection. Graph shows to maintenance of decreased central macular thickness until 2 years later (p = 0.01, Wilcoxon signed rank test). |
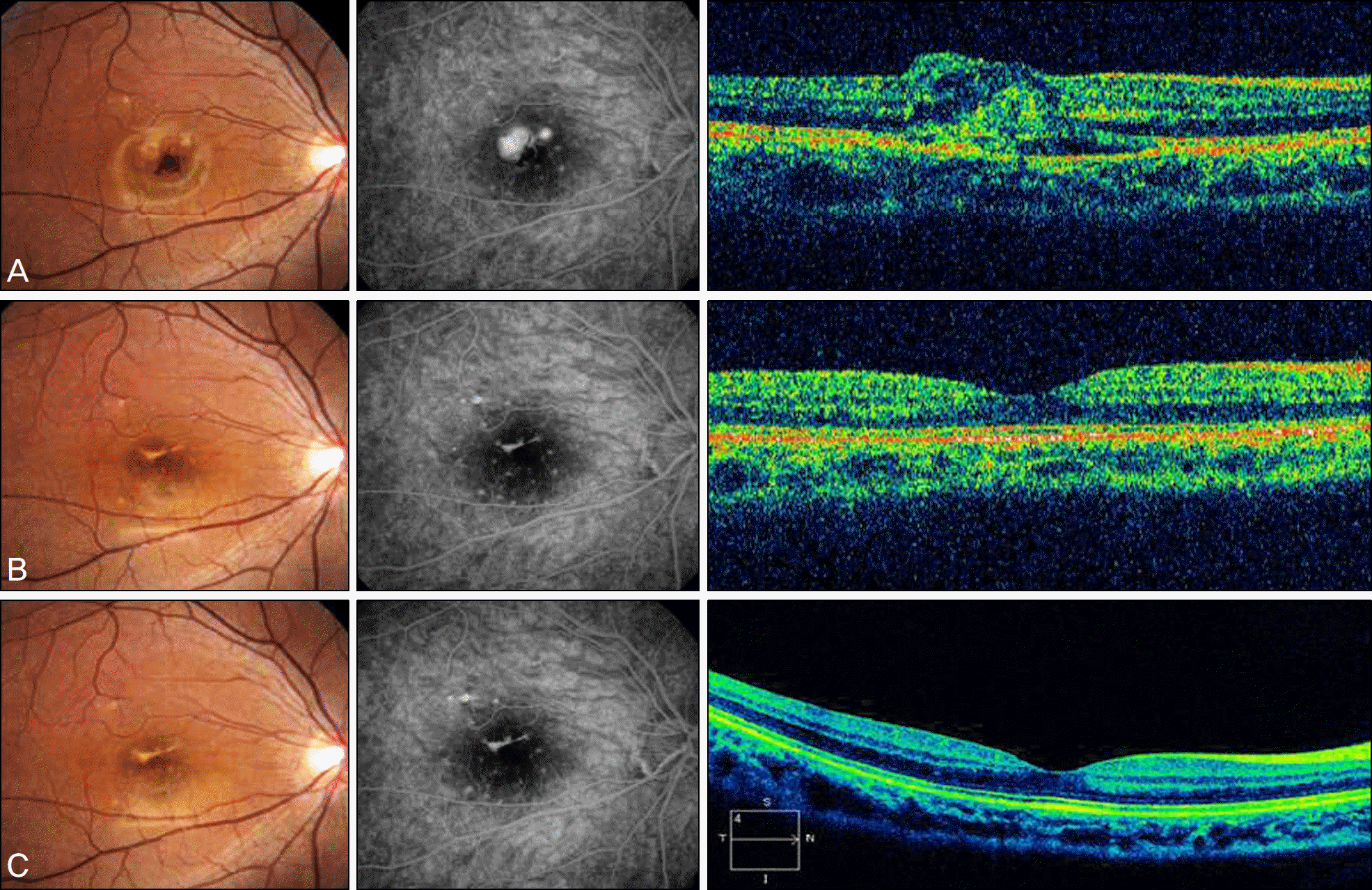 | Figure 4.Serial fundus photographs and fluorescein angiographs and optical coherence tomographs of case 6. (A) images before intravitreal bevacizumab injection showed subfoveal hemorrhage and juxtafoveal classic CNV and macular edema. At this time BCVA was 0.02 (logMAR 1.70). At 1 year after intravitreal bevacizumab injection, (B) images showed scar change of CNV membrane and just fluorescein staining without fluorescein leakage. BCVA at 1 year was 1.0 (logMAR 0.00). At 2 years after intravitreal bevacizumab injection, (C) images showed similar findings at (B) images. BCVA at 2 year was 0.9 (logMAR 0.04). Until two years later, very good and stable prognosis has been shown. |
Table 1.
Patient characteristics
| Pt. case no. | e Sex | Age | eye | Refractive error (diopters) |
BCVA† (log MAR‡) |
location |
CMT§ (μ m) |
No. of injections | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Month 0 | 1 | 3 | 12 | 24 | Month 0 | 24 | |||||||
| 1 | F | 46 | L | −12 | 0.70 | 0.30 | 0.15 | 0.20 | 0.15 | Subfovea | 440 | 275 | 1 |
| 2 | M | 44 | R | −9.25 | 1.10 | 0.30 | 0.04 | 0.04 | 0.04 | Subfovea | 300 | 269 | 2 |
| 3* | M | 55 | L | −13 | 0.80 | 0.70 | 0.70 | 0.30 | 0.10 | Subfovea | 295 | 279 | 1 |
| 4 | M | 51 | L | −8 | 1.00 | 0.20 | 0.15 | 0.15 | 0.15 | Subfovea | 350 | 240 | 2 |
| 5 | F | 20 | L | −11 | 1.70 | 0.50 | 0.30 | 0.20 | 0.20 | Subfovea | 298 | 181 | 1 |
| 6 | F | 15 | R | −9 | 1.70 | 0.70 | 0.40 | 0.00 | 0.04 | juxtafovea | 420 | 244 | 1 |
| Mean | | 38.5 | | −10.37 | 1.16 | 0.45 | 0.29 | 0.14 | 0.11 | | 350.5 | 248 | 1.33 |
| SD | | 16.7 | | 1.93 | 0.43 | 0.21 | 0.23 | 0.11 | 0.06 | | 65.1 | 36.6 | 0.51 |
| | F:3 | | R;2 | | | | | | | Subfovea:5 | | | |
| | M:3 | | L:4 | | | | | | | Juxtafovea:1 | | | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download