Abstract
Purpose
To investigate the role of gangliosides in the differentiation of orbital fibroblasts into adipocytes, a component in the pathogenesis of Graves’ ophthalmopathy.
Methods
Orbital tissues were obtained during orbital surgery for subjects without Graves’ ophthalmopathy or other inflammatory orbital disease, and orbital fibroblasts were primarily cultured from each obtained tissue. Morphological examination of orbital fibroblasts was performed after treatment with commercially available gangliosides mixture (Gmix) comprised of several subtypes. To determine the effect of Gmix on the differentiation of orbital fibroblasts into adipocytes and the differentiation-related genes, Oil Red-O staining and RT-PCR were performed.
Results
The treatment with Gmix induced the morphological changes, which at least in part were explained with the differentiation of orbital fibroblasts into adipocytes in accordance with the increase of mRNA level of genes known to be related to adipogenesis, whereas dermal fibroblasts and preadipocytes were irresponsive to the same treatment.
References
1. Prabhakar BS, Bahn RS, Smith TJ. Current perspective on the pathogenesis of Graves’ disease and ophthalmopathy. Endocr Rev. 2003; 24:802–35.

3. Forbes G, Gorman CA, Brennan MD, et al. Ophthalmopathy of Graves’ disease: computerized volume measurements of the orbital fat and muscle. AJNR Am J Neuroradiol. 1986; 7:651–6.
4. Tallstedt L, Norberg R. Immunohistochemical staining of normal and Graves’ extraocular muscle. Invest Ophthalmol Vis Sci. 1988; 29:175–84.
5. Kahaly G, Forster G, Hansen C. Glycosaminoglycans in thyroid eye disease. Thyroid. 1998; 8:429–32.

6. Hufnagel TJ, Hickey WF, Cobbs WH, et al. Immunohistochemical and ultrastructural studies on the exenterated orbital tissues of a patient with Graves’ disease. Ophthalmology. 1984; 91:1411–9.

7. Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as senti-nel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997; 151:317–22.
8. Fries KM, Blieden T, Looney RJ, et al. Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin Immunol Immunopathol. 1994; 72:283–92.

9. Cao HJ, Smith TJ. Leukoregulin upregulation of prostaglandin en-doperoxide H synthase-2 expression in human orbital fibroblasts. Am J Physiol. 1999; 277:C1075–85.

10. Smith TJ, Bahn RS, Gorman CA, Cheavens M. Stimulation of gly-cosaminoglycan accumulation by interferon gamma in cultured human retroocular fibroblasts. J Clin Endocrinol Metab. 1991; 72:1169–71.

11. Smith TJ, Koumas L, Gagnon A, et al. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2002; 87:385–92.

13. Ruan S, Raj BK, Lloyd KO. Relationship of glycosyltransferases and mRNA levels to ganglioside expression in neuroblastoma and melanoma cells. J Neurochem. 1999; 72:514–21.

14. Tsuji S. Molecular cloning and functional analysis of sialyltran-sferases. J Biochem. 1996; 120:1–13.

15. Hakomori Si SI. The glycosynapse. Proc Natl Acad Sci USA. 2002; 99:225–32.
16. Nojiri H, Takaku F, Terui Y, et al. Ganglioside GM3: an acidic membrane component that increases during macrophage-like cell differentiation can induce monocytic differentiation of human myeloid and monocytoid leukemic cell lines HL-60 and U937. Proc Natl Acad Sci USA. 1986; 83:782–6.

17. Hakomori S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 1990; 265:18713–6.

18. Hakomori S, Yamamura S, Handa AK. Signal transduction through glyco(sphingo)lipids. Introduction and recent studies on glycol (sphingo)lipid-enriched microdomains. Ann N Y Acad Sci. 1998; 845:1–10.
19. Mullin BR, Fishman PH, Lee G, et al. Thyrotropin-ganglioside interactions and their relationship to the structure and function of thyrotropin receptors. Proc Natl Acad Sci USA. 1976; 73:842–6.

20. Aloj SM, Kohn LD, Lee G, Meldolesi MF. The binding of thyrotropin to liposomes containing gangliosides. Biochem Biophys Res Commun. 1977; 74:1053–9.

21. Kielczynski W, Harrison LC, Leedman PJ. Direct evidence that ganglioside is an integral component of the thyrotropin receptor. Proc Natl Acad Sci USA. 1991; 88:1991–5.

22. Kielczynski W, Bartholomeusz RK, Harrison LC. Characterization of ganglioside associated with the thyrotrophin receptor. Glycobi-ology. 1994; 4:791–6.

23. Bouchon B, Portoukalian J, Bornet H. Major gangliosides in normal and pathological human thyroids. Biochem Int. 1985; 10:531–8.
24. Kiljanski J, Ambroziak M, Pachucki J, et al. Thyroid sialyltransfer-ase mRNA level and activity are increased in Graves' disease. Thyroid. 2005; 15:645–52.

25. Sorisky A, Pardasani D, Gagnon A, Smith TJ. Evidence of adipocyte differentiation in human orbital fibroblasts in primary culture. J Clin Endocrinol Metab. 1996; 81:3428–31.

26. Reddy L, Wang HS, Keese CR, et al. Assessment of rapid morphological changes associated with elevated cAMP levels in human orbital fibroblasts. Exp Cell Res. 1998; 245:360–7.

27. Valyasevi RW, Jyonouchi SC, Dutton CM, et al. Effect of tumor necrosis factor-alpha, interferon-gamma, and transforming growth factor-beta on adipogenesis and expression of thyrotropin receptor in human orbital preadipocyte fibroblasts. J Clin Endocrinol Metab. 2001; 86:903–8.
28. Valyasevi RW, Harteneck DA, Dutton CM, Bahn RS. Stimulation of adipogenesis, peroxisome proliferator-activated receptor-gamma (PPARgamma), and thyrotropin receptor by PPARgamma agonist in human orbital preadipocyte fibroblasts. J Clin Endocrinol Metab. 2002; 87:2352–8.
29. Feldon SE, O'Loughlin CW, Ray DM, et al. Activated human T lymphocytes express cyclooxygenase-2 and produce proadipo-genic prostaglandins that drive human orbital fibroblast differentiation to adipocytes. Am J Pathol. 2006; 169:1183–93.

31. Chen B, Tsui S, Smith TJ. IL-1 beta induces IL-6 expression in human orbital fibroblasts: identification of an anatomic-site specific phenotypic attribute relevant to thyroid-associated ophthalmopathy. J Immunol. 2005; 175:1310–9.
32. Young DA, Evans CH, Smith TJ. Leukoregulin induction of protein expression in human orbital fibroblasts: evidence for anatomical site-restricted cytokine-target cell interactions. Proc Natl Acad Sci USA. 1998; 95:8904–9.

33. Smith TJ, Sempowski GD, Wang HS, et al. Evidence for cellular heterogeneity in primary cultures of human orbital fibroblasts. J Clin Endocrinol Metab. 1995; 80:2620–5.

Figure 1.
The effect of gangliosides mixture (Gmix) treatment on morphological changes of orbital fibroblasts. On phase contrast microscopic examination, Gmix induced morphological changes of orbital fibroblasts in dose-dependent manner up to 100 µg/mL of concentration at 48 hours of treatment.
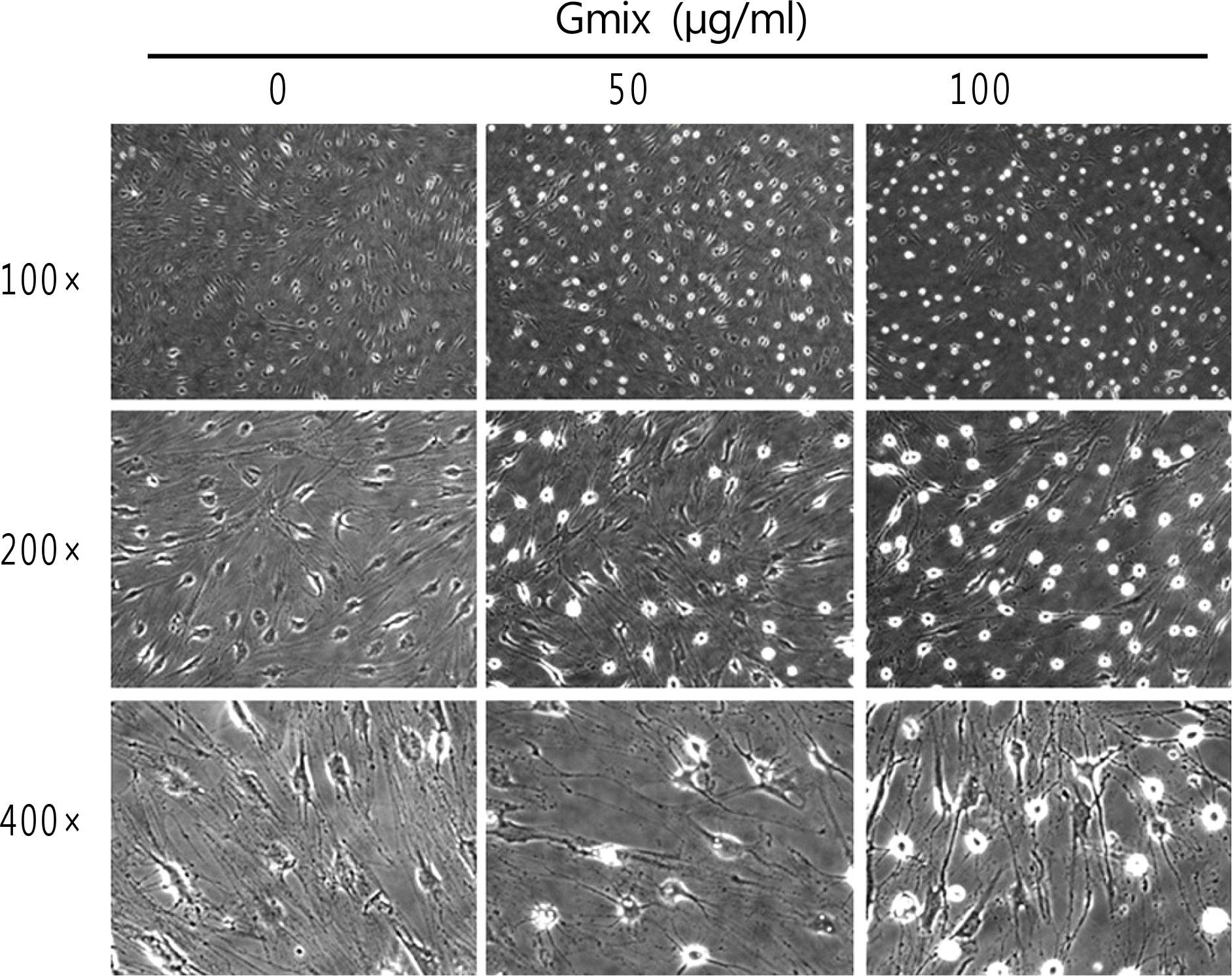
Figure 2.
The effect of gangliosides mixture (Gmix) treatment on morphological changes in dermal fibroblast and preadipocyte. Phase contrast microscopic examination of dermal fibroblasts and preadipocyte cell line (3T3-L1) after treatment with 100 µg/mL of Gmix was performed. No detectable morphological changes are noted up to 48 hours compared to untreated control (×400).
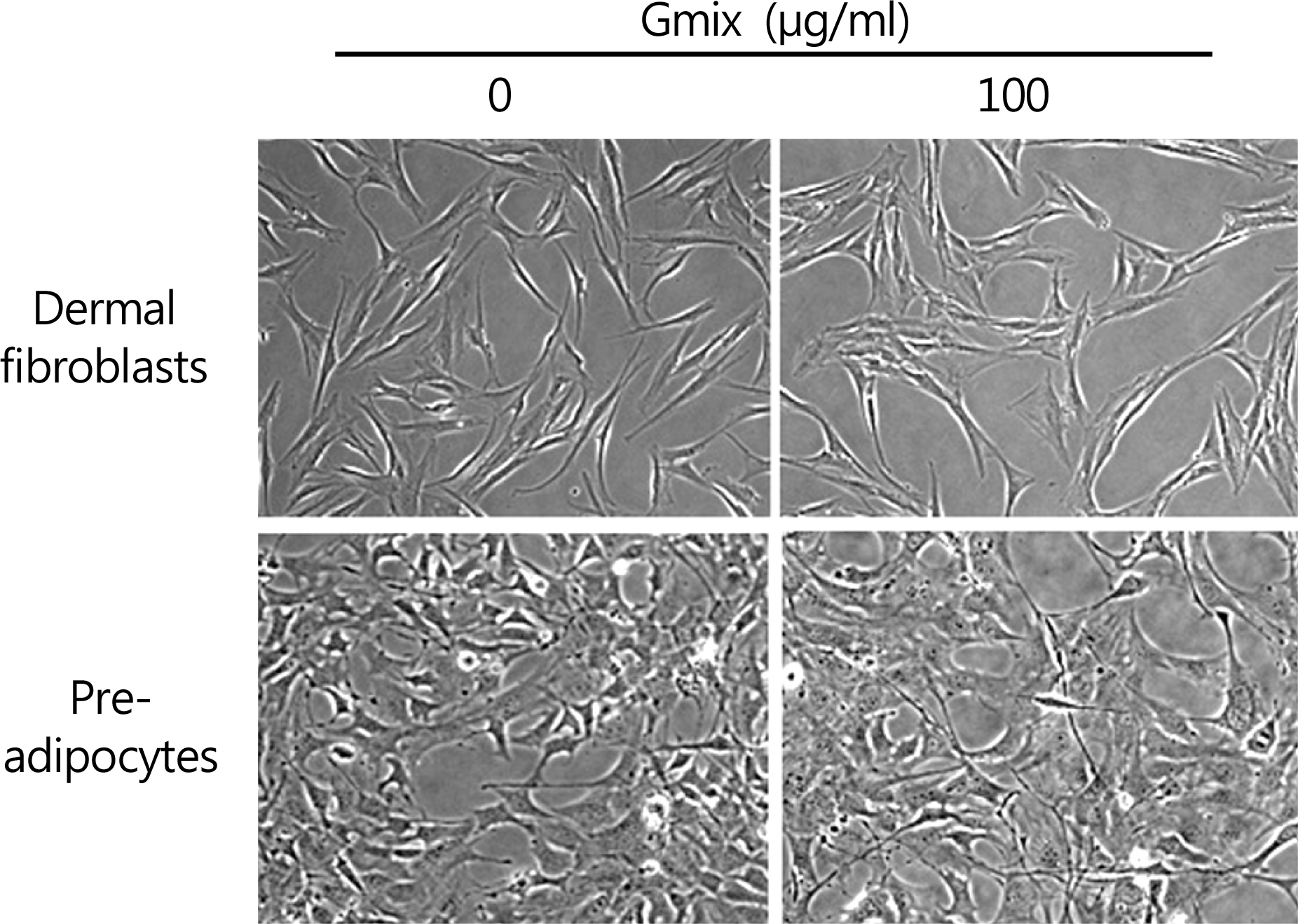
Figure 3.
Differentiation of orbital fibroblast into adipocyte by gangliosides mixture (Gmix) treatment. (A) Oil red O staining of orbital fibroblast. Oil red O stain was performed at 48 hours after treatment with 100 µg/ml of Gmix. More and larger lipid droplets are noted in Gmix-treated orbital fibroblasts than untreated control, and they are stained with red color by oil red O staining. (B) Density of oil red O extracted from experiment A.
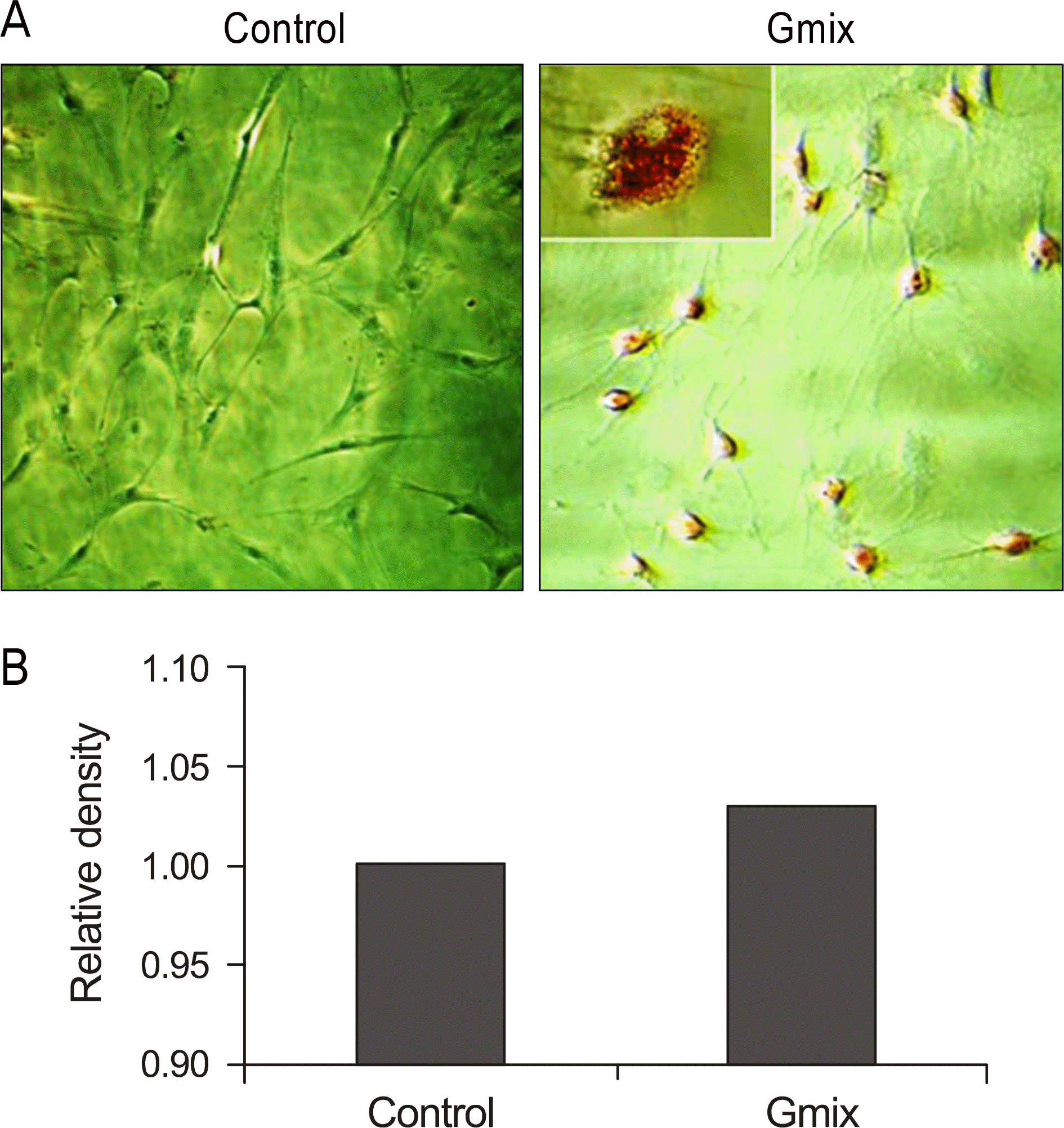
Figure 4.
RT-PCR for C/EBPβ and PPARγ1 from orbital fibroblasts following treatment with gangliosides mixture (Gmix) at various time points. Increased induction of C/EBPβ as well as PPARγ1 expression is noted evidently at 24 and 48 hours after 100 µg/mL of Gmix treatment.
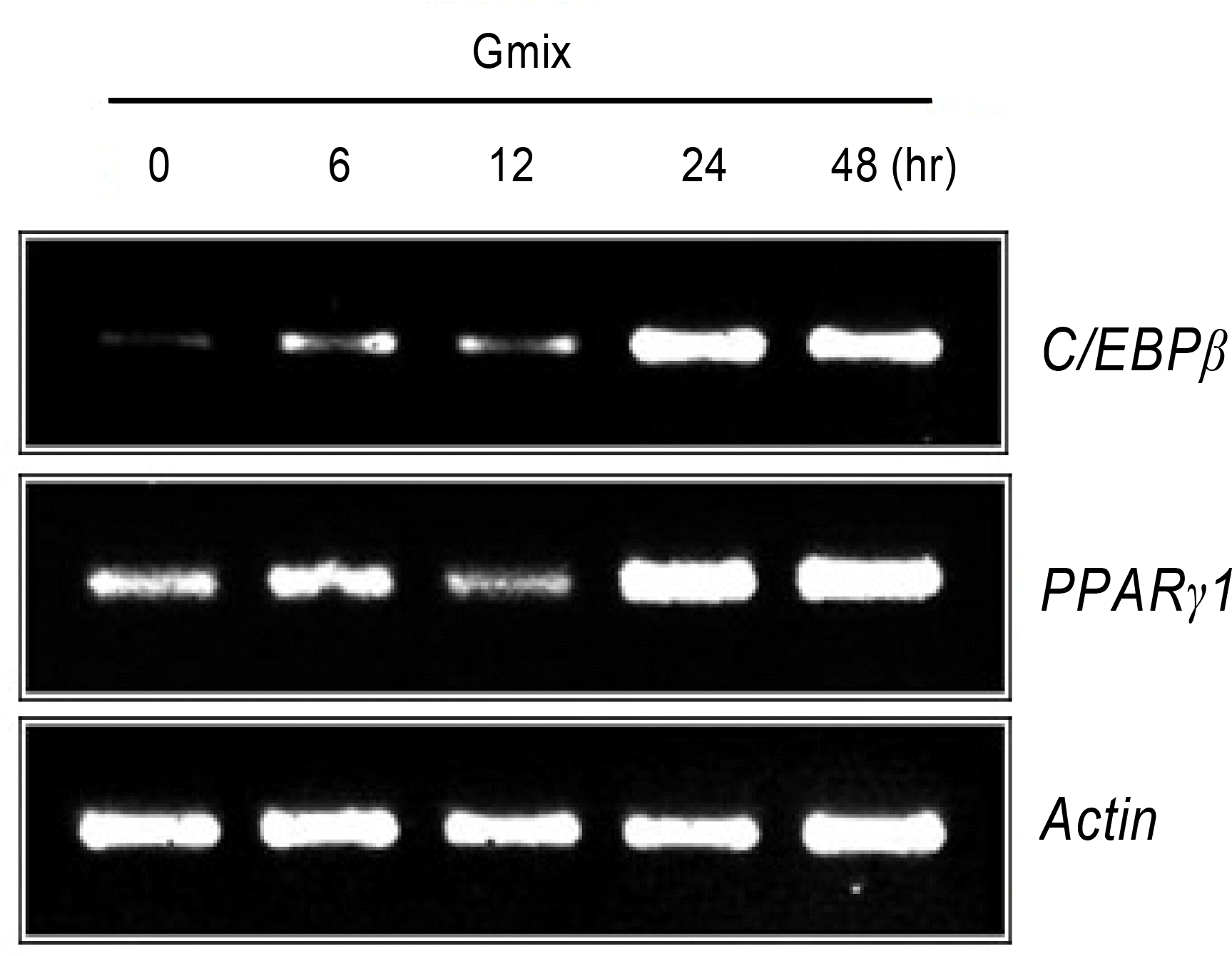




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download