Abstract
Methods
This is a retrospective study of 125 patients who were referred to our clinic and diagnosed with GO from March 2006 to August 2008. Ophthalmic examinations and a thyroid function test including TSI were performed at the time of diagnosis and every 3 months after diagnosis. All patients were classified as having a mild or severe course of GO, according to Clinical Activity Score (CAS) and NOSPECS classifications.
Results
This statistical analysis of each group revealed that female sex (p = 0.04), age (p = 0.0004), smoking (p = 0.003), and high TSI level (p = 0.009) were significant factors for severe course of GO at the time of diagnosis. TSI levels at each of the 3 visits during the followup were significantly higher in patients with a severe course of GO, as compared to patients with a mild course of GO (first visit: p = 0.003, second visit: p = 0.002, third visit: p = 0.006).
Go to : 
References
1. Burch HB, Wartofsky L. Graves’ ophthalmopathy: current concepts regarding pathogenesis and management. Endocr Rev. 1993; 14:747–93.

2. Asman P. Ophthalmological evaluation in thyroid-associated ophthalmopathy. Acta Ophthalmol Scand. 2003; 81:437–48.
3. Brix TH, Kyvik KO, Hegedüs L. What is the evidence of genetic factors in the etiology of Graves’ disease? A brief review. Thyroid. 1998; 8:727–34.

4. Weetman AP, McGregor AM. Autoimmune thyroid disease: further developments in our understanding. Endocr Rev. 1994; 15:788–830.

5. Hägg E, Asplund K. Is endocrine ophthalmopathy related to smoking? Br Med J. 1987; 295:634–5.
7. Bahn RS. Understanding the immunology of Graves’ ophthalmopathy. Is it an autoimmune disease? Endocrinol Metab Clin North Am. 2000; 29:287–96.

8. Bahn RS, Dutton CM, Natt N, et al. Thyrotropin receptor expression in Graves’ orbital adipose/connective tissues: potential autoantigen in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1998; 83:998–1002.

9. Eckstein AK, Plicht M, Lax H, et al. Thyrotropin receptor autoanti-bodies are independent risk factors for Graves’ ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab. 2006; 91:3464–70.

10. Chae JK, Kim BJ, Michael K. The risk factors for severe thy-roid-associated orbitopathy. J Korean Ophthalmol Soc. 2006; 47:1238–43.
11. Wakelkamp IM, Gerding MN, van der Meer JW, et al. Smoking and disease severity are independent determinants of serum adhesion molecule levels in Graves’ ophthalmopathy. Clin Exp Immunol. 2002; 127:316–20.

12. Tallstedt L, Lundell G, T⊘rring O, et al. Occurrence of ophthalmopathy after treatment for Graves’ hyperthyroidism. The Thyroid Study Group. N Engl J Med. 1992; 326:1733–8.
13. Bartalena L, Marcocci C, Bogazzi F, et al. Relation between therapy for hyperthyroidism and the course of Graves’ ophthalmopathy. N Engl J Med. 1998; 338:73–8.

14. Atkinson S, McGregor AM, Kendall-Taylor P, et al. Effect of radio-iodine on stimulatory activity of Graves’ immunoglobulins. Clin Endocrinol. 1982; 16:537–43.
15. Prummel MF, Wiersinga WM, Mourits MP, et al. Effect of abnormal thyroid function on the severity of Graves’ ophthalmopathy. Arch Intern Med. 1990; 150:1098–101.

16. Benker G, Kotulla P, Kendall-Taylor P, et al. TSH binding-inhibiting antibodies in hyperthyroidism: relationship to clinical signs and hormone levels. Clin Endocrinol. 1989; 30:19–28.

17. Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves’ ophthalmopathy. Clin Endocrinol. 1997; 47:9–14.

18. Wiersinga WM, Prummel MF, Mourits MP, et al. Classification of the eye changes of Graves’ disease. Thyroid. 1991; 1:357–60.

19. Bartalena L, Marcocci C, Tanda ML, et al. Cigarette smoking and treatment outcomes in Graves ophthalmopathy. Ann Intern Med. 1998; 129:632–5.

20. Eckstein A, Quadbeck B, Mueller G, et al. Impact of smoking on the response to treatment of thyroid associated ophthalmopathy. Br J Ophthalmol. 2003; 87:773–6.

21. Marcocci C, Bruno-Bossio G, Manetti L, et al. The course of Graves’ ophthalmopathy is not influenced by near total thyroidectomy: a case-control study. Clin Endocrinol. 1999; 51:503–8.

22. Järhult J, Rudberg C, Larsson E, et al. Graves'disease with moder-ate-severe endocrine ophthalmopathy-long term results of a prospective, randomized study of total or subtotal thyroid resection. Thyroid. 2005; 15:1157–64.
Go to : 
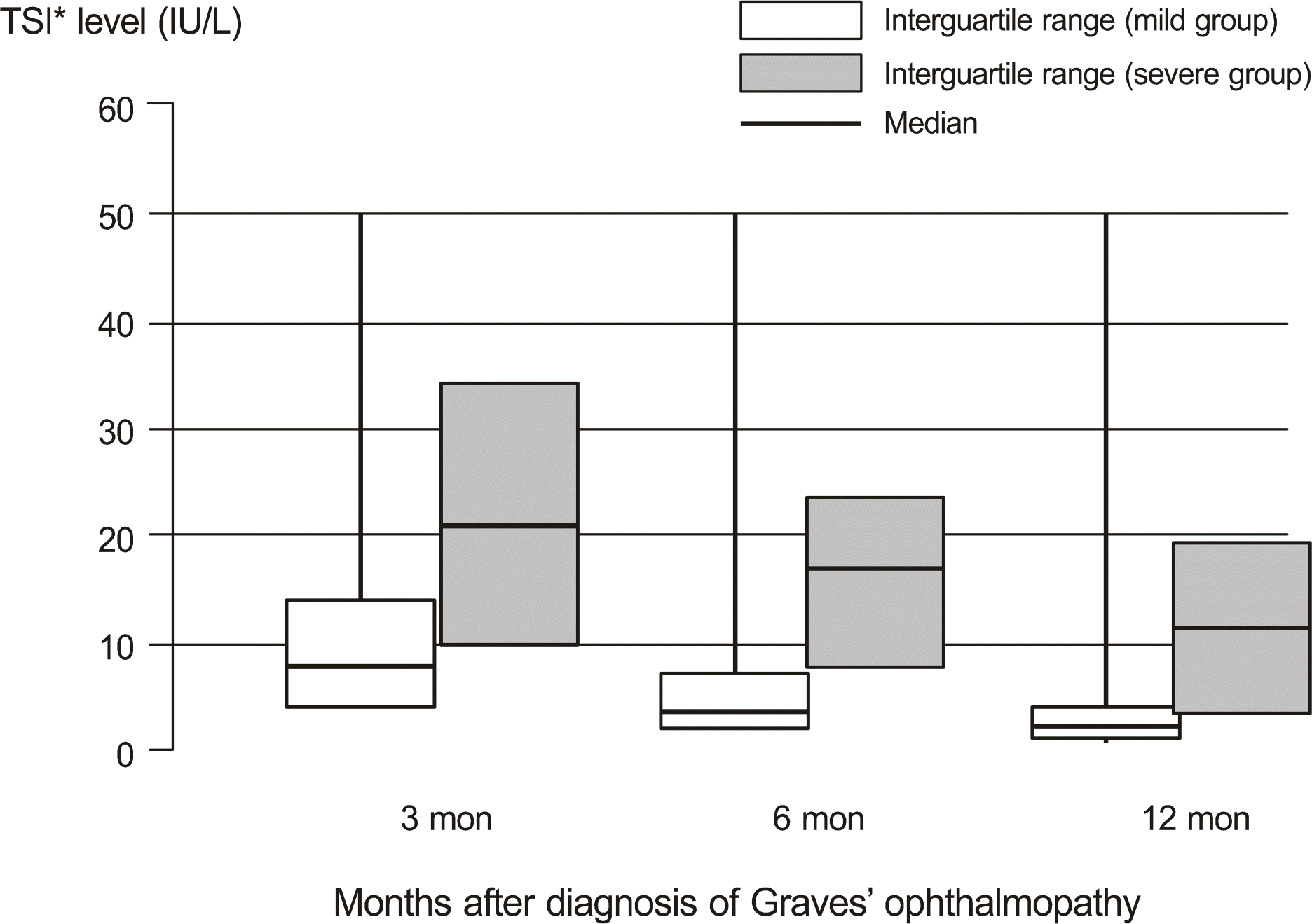 | Figure 1.Association between TSI∗ level and the severity. ∗ TSI = TSH stimulating immunoglobin. |
Table 1.
Characteristics of patients with Graves’ ophthalmopathy (n∗ = 125)
| Patient characteristics | N∗/mean ± SD† | (%) |
|---|---|---|
| Study population | 125 | 100 |
| Mean age ± SD | 49.6 ± 9.8 | |
| Sex (male/female) | 52/73 | 41.6/58.4 |
| Smoker | 48 | 38.4 |
| Treatment for ophthalmopathy | ||
| No treatment | 10 | 8.0 |
| Conservative treatment | 59 | 47.2 |
| High-dose IV‡ pulse steroid therapy | 45 | 36.0 |
| Oral steroid therapy | 38 | 30.4 |
| Orbital decompression | 4 | 3.2 |
| Severity of GO§ | ||
| Mild group | 85 | 68 |
| Severe group | 40 | 32 |
| Thyroid function | ||
| Hyperthyroid | 28 | 22.4 |
| Euthyroid | 49 | 39.2 |
| Hypothyroid | 11 | 8.8 |
| Others | 37 | 29.6 |
| Treatments for thyroid dysfunction | ||
| Antithyroid medication | 60 | 48 |
| Thyroidectomy | 17 | 13.6 |
| Radioiodine treatment | 11 | 8.8 |
| Thyroid hormone | 33 | 26.4 |
Table 2.
Distribution of Clinical Activity Score (CAS) and NOSPECS classification among the Graves’ ophthalmopathy patients
| CAS∗ | n† (%) | NOSPECS‡ | n (%) |
|---|---|---|---|
| 0 | 5 (4.0) | 0 | 1 (0.8) |
| 1 | 37 (29.6) | 1 | 4 (3.2) |
| 2 | 32 (25.6) | 2 | 15 (12.0) |
| 3 | 27 (21.6) | 3 | 47 (37.6) |
| 4 | 23 (18.4) | 4 | 20 (16.0) |
| 5 | 8 (6.4) | 5 | 31 (24.8) |
| 6 | 5 (4.0) | 6 | 2 (1.6) |
| 7 | 2 (1.6) | >6 | 5 (4.0) |
| Total | 125 (100) | Total | 125 (100.0) |
Table 3.
Risk factors with influence on the severity of Graves’ ophthalmopathy
| Risk factor | p-value∗ | Risk ratio (95% CI) |
|---|---|---|
| TSI† level | 0.0090 | 1.36‡ |
| Smoking | 0.0030 | 8.12 |
| Sex (female) | 0.0400 | 4.62 |
| Age | 0.0004 | 1.12 |
| Radioiodine treatment | 0.2300 | 1.72 |
| Thyroidectomy treatment | 0.5600 | 1.65 |
| Free T4 level (ng/dL) | 0.8000 | 1.11 |
| TSH§ level (uIU/mL) | 0.7000 | 1.05 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download