Abstract
Purpose
To investigate the effects of two antimetabolites, mitomycin C (MMC) and 5-fluorouracil (5-FU), on proliferation of cultured human nasal mucosa fibroblasts.
Methods
Human nasal mucosa fibroblasts were primarily cultured, and exposed to various concentrations of MMC and 5-FU for 5 minutes. Control fibroblasts were exposed to only DMEM media without the drugs. Effect of drugs on cell morphology was observed by phase-contrast microscopy. Cell viability and apoptosis were measured using MTT [3-(4,5-dime-thylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide] assay and Acridine orange/Hoechst (AO/HO) staining, respectively.
Go to : 
References
1. Caldwell GW. Two new operations for the radical cure of obstruction of the nasal duct with preservation of the canalicuoli and an incidental description of a new lacrimal probe. NY Med J. 1893; 57:581.
2. McDonogh M, Meiring JH. Endoscopic transnasal dacryocystorhinostomy. J Laryngol Otol. 1989; 103:585–7.

3. Woog JJ, Kennedy RH, Custer PL, et al. Endonasal dacryocystorhinostomy: a report by the American Academy of Ophthalmology. Ophthalmology. 2001; 108:2369–77.
4. Lee HC, Chung WS. Success rate of endonasal Dacryocystorhinostomy. J Korean Ophthalmol Soc. 1996; 37:211–8.
5. Lee SH, Chung WS. Long term surgical efficacy of endonasal dacryocystorhinostomy. J Korean Ophthalmol Soc. 2000; 41:307–13.
6. Lee TS, Kim JS, Kim JK. The effect of double silicone tube intubation on surgical outcome of endonasal dacryocystorhinostomy. J Korean Ophthalmol Soc. 2002; 43:2089–94.
7. Allen K, Berlin AJ. Dacryocystorhinostomy failure: association with nasolacrimal silicone intubation. Ophthalmic Surg. 1989; 20:486–9.

8. Zolli CL, Shannon GM. Dacryocystorhinostomy: a review of 119 cases. Ophthalmic Surg. 1982; 13:905–10.

9. McLachlan DL, Shannon GM, Flanagan JC. Results of dacryocystorhinostomy: analysis of the reoperation. Ophthalmic Surg. 1980; 11:427–30.
10. Tarbet KJ, Custer PL. External dacryocystorhinostomy. Surgical success, patient satisfaction, and economic cost. Ophthalmology. 1995; 102:1065–70.
11. Walland MJ, Rose GE. Factors affecting the success rate of open lacrimal surgery. Br J Ophthalmol. 1994; 78:888–91.

12. Kao SC, Liao CL, Tseng JH, et al. Dacryocystorhinostomy with intraoperative mitomycin C. Ophthalmology. 1997; 104:86–91.

13. Yalaz SC, Firinciogullari E, Zeren H. Use of mitomycin C and 5-fluorouracil in external dacryocystorhinostomy. Orbit. 1999; 18:239–45.

14. Kim JH, Kim KS, Yoon HC, et al. Anti-adhesive effect of GUARDIX-SL® after endoscopic sinus surgery. Korean J Otolaryngol- Head Neck Surg. 2005; 48:1478–83.
15. Lee TS, Lee KC. The effect of Mitomycin-C eyedrop on pre-vention of internal ostium obstruction after endonasal dacryocystorhinostomy. J Korean Ophthalmol Soc. 1998; 39:1915–20.
16. Ugurbas SH, Zilelioglu G, Sargon MF, et al. Histopathologic effects of mitomycin-C on endoscopic transnasal dacryocystorhinostomy. Ophthalmic Surg Lasers. 1997; 28:300–4.

17. Zilelioğ lu G, Uğ urbaş SH, Anadolu Y, et al. Adjunctive use of mitomycin C on endoscopic lacrimal surgery. Br J Ophthalmol. 1998; 82:63–6.
18. Roozitalab MH, Amirahmadi M, Namazi MR. Results of the application of intraoperative mitomycin C in dacryocystorhinostomy. Eur J Ophthalmol. 2004; 14:461–3.

19. Iliff CE. A simplified dacryocystorhinostomy. 1954-1970. Arch Ophthalmol. 1971; 85:586–91.
20. Watts P, Ram AR, Nair R, Williams H. Comparison of external dacryocystorhinostomy and 5-fluorouracil augmented endonasal laser dacryocystorhinostomy. A retrospective review. Indian J Ophthalmol. 2001; 49:169–72.
21. Hu D, Sires BS, Tong DC, et al. Effect of brief exposure to mitomycin C on cultured human nasal mucosa fibroblasts. Ophthal Plast Reconstr Surg. 2000; 16:119–25.

22. Givens KT, Kitada S, Chen AK, et al. Proliferation of human ocular fibroblasts. An assessment of in vitro colorimetric assays. Invest Ophthalmol Vis Sci. 1990; 31:1856–62.
23. Senderoff RI, Weber PA, Smith DR, Sokoloski TD. Evaluation of antiproliferative agents using a cell-culture model. Invest Ophthalmol Vis Sci. 1990; 31:2572–8.
24. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.

25. Mpoke SS, Wolfe J. Differential staining of apoptotic nuclei in living cells: Application to macronuclear elimination in Tetrahymena. J Histochem Cytochem. 1997; 45:675–83.
26. Gilman AG, Goodman LS, Gilman A. The Pharmacological Basis of Therapeutics. 6th ed.New York: Macmillan Publishing Co.;1980. p. 1295–300.
27. Dushinski R, Pleven E, Heidelberger C. The synthesis of 5 fluoro pyrimidines. J Am Chem Soc. 1957; 79:4559–60.
28. Blumenkranz MS, Ophir A, Claflin AJ, Hajek A. Fluorouracil for the treatment of massive periretinal proliferation. Am J Ophthalmol. 1982; 94:458–67.

29. Abraham LM, Selva D, Casson R, Leibovitch I. The clinical appli cations of fluorouracil in ophthalmic practice. Drugs. 2007; 67:237–55.
30. Yamamoto T, Varani J, Soong HK, Lichter PR. Effects of 5-fluo-rouracil and mitomycin C on cultured rabbit subconjunctival fibroblasts. Ophthalmology. 1990; 97:1204–10.

31. Singh K, Mehta K, Shaikh NM, et al. Trabeculectomy with intraoperative mitomycin C versus 5-fluorouracil. Prospective randomized clinical trial. Ophthalmology. 2000; 107:2305–9.
32. Suh WJ, Chung WS. Effects of mitomycin C on cultured rabbit osteoblasts. J Korean Ophthalmol Soc. 2001; 42:1464–9.
33. Lee JH, Chung WS, Son JH. The effect of mitomycin C on cultured human osteoblasts. J Korean Ophthalmol Soc. 2003; 44:2122–7.
34. Huang L, Wong YP, Cai YJ. Low dose 5-fluorouracil induces cell cycle G2 arrest and apoptosis in keloid fibroblasts. Br J Dermatol. 2010; 163:1181–5.
Go to : 
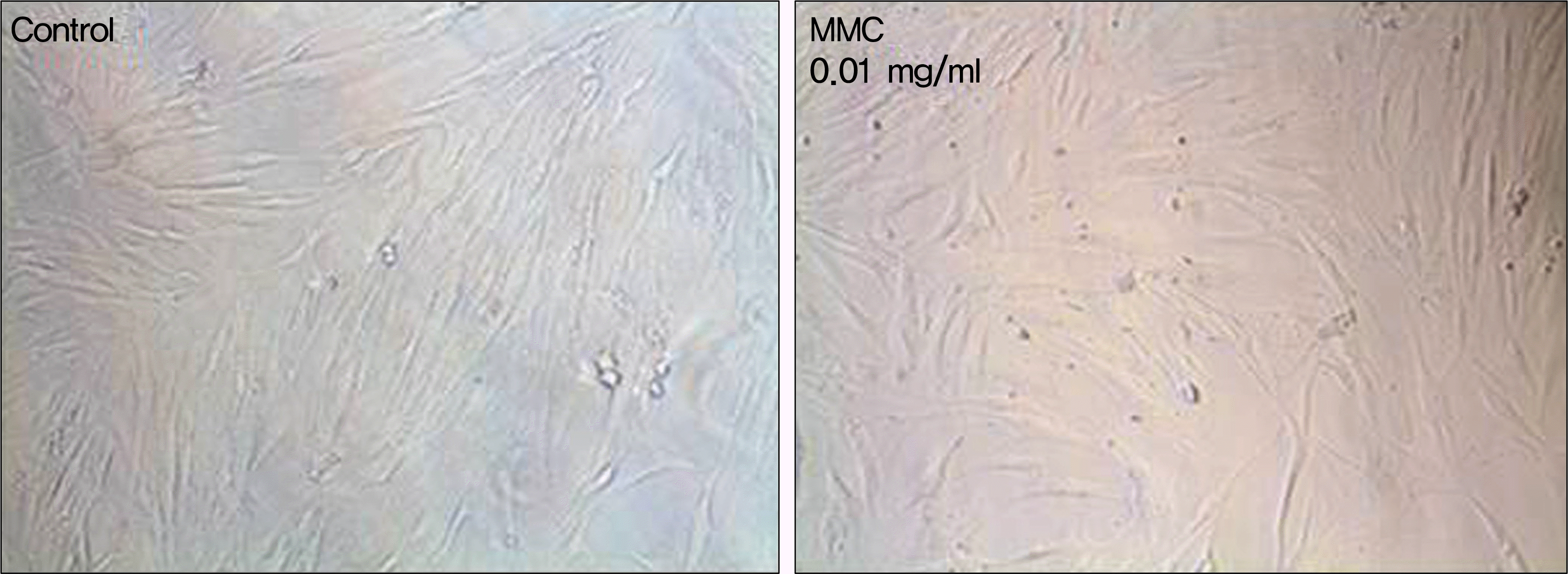 | Figure 1.The morphology of cultured human nasal mucosa fibroblasts under phase-contrast microscope. Fibroblasts were treated with various concentrations of MMC for 5 minutes and then incubated. Images are representatives of day 1 of culture. The viable cells were spindle-shaped at all concentrations of MMC. Magnification, ×100. MMC = mitomycin C. |
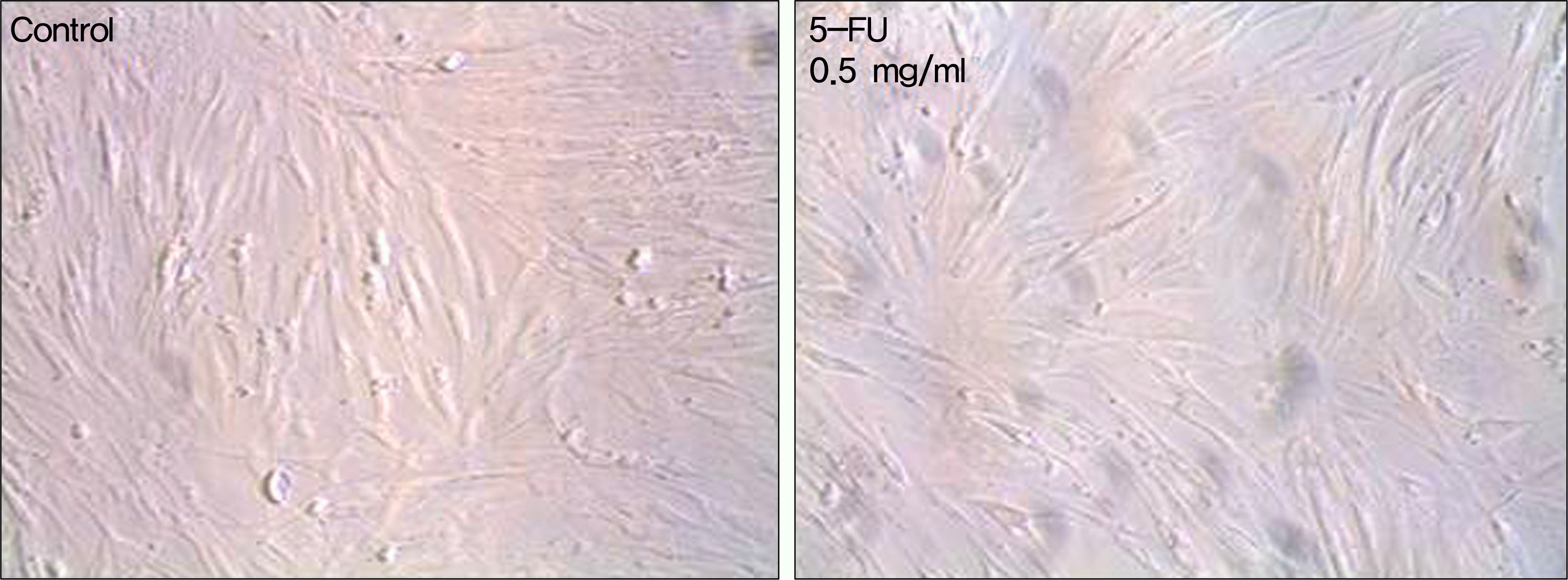 | Figure 2.The morphology of cultured human nasal mucosa fibroblasts under phase-contrast microscope. Fibroblasts were treated with various concentrations of 5-FU for 5 minutes and then incubated. Images are representatives of day 1 of culture. The viable cells were spindle-shaped at all concentrations of 5-FU. Magnification, ×100. 5-FU=5-fluorouracil. |
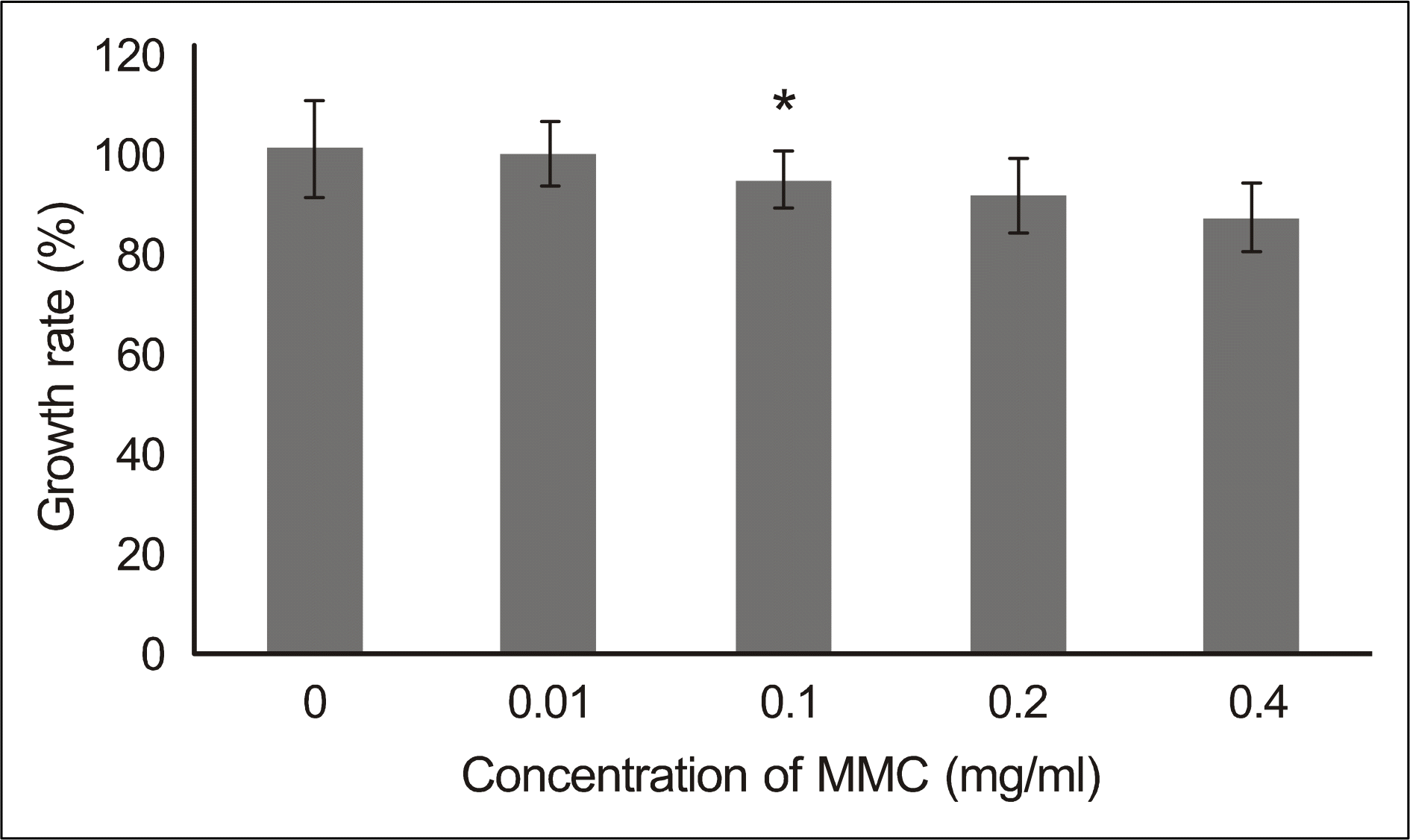 | Figure 3.The viability of the cultured human nasal mucosa fibroblasts treated with various concentrations of MMC. Fibroblasts were treated for 5 minutes with MMC (0.01, 0.1, 0.2, 0.4 mg/ml), washed and further incubated for 24 hr. Optical density (OD) of the fibroblasts was measured using MTT assay. Growth rates (%) were calculated as [100 × (OD values of drug-treated cells/OD values of non-treated cells)]. Values and bars represent means of growth rates and the SD, respectively. ∗ p<0.05 compared to non-treated control. MMC = mitomycin C. |
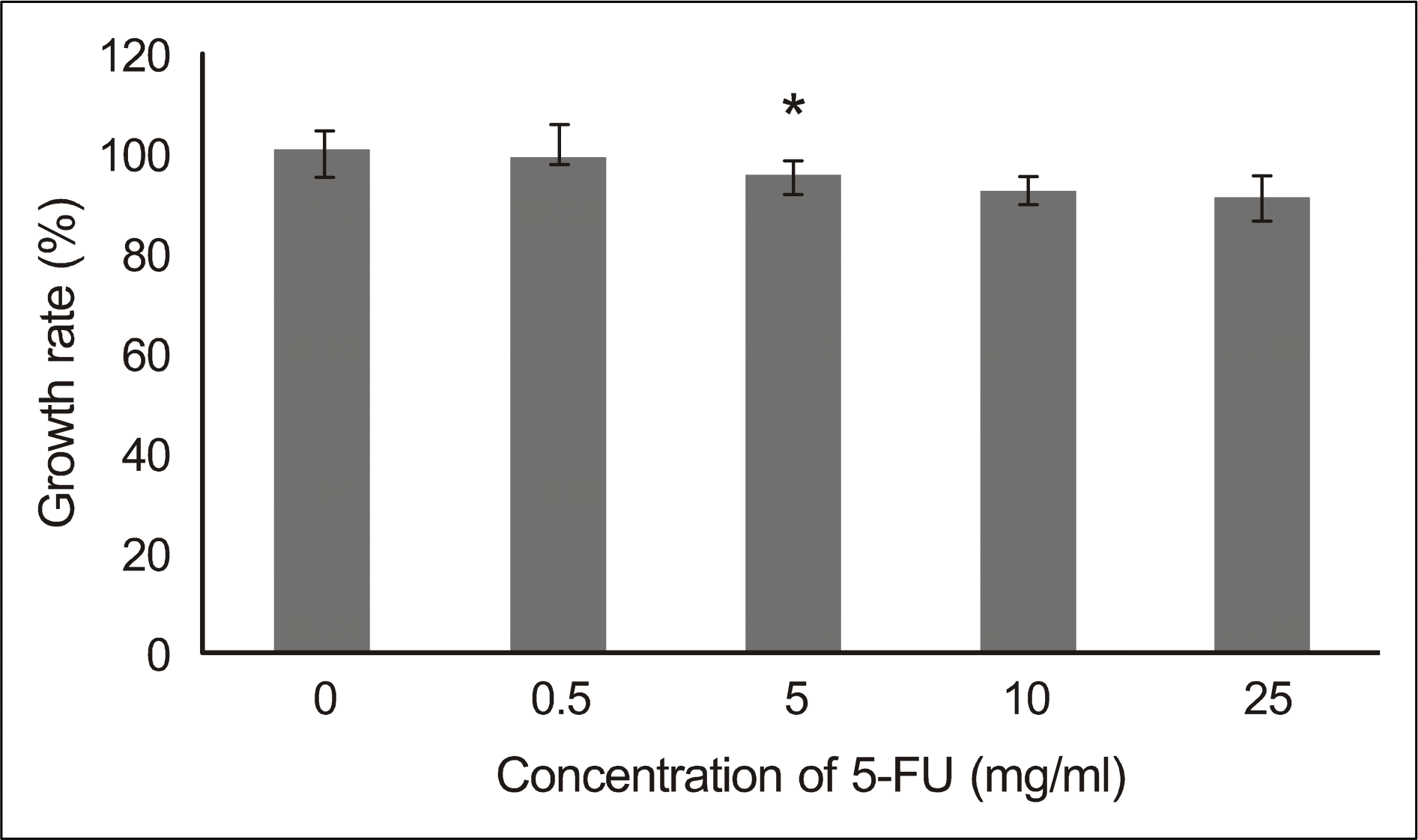 | Figure 4.The viability of the cultured human nasal mucosa fibroblasts treated with various concentrations of 5-FU. Fibroblasts were treated for 5 minutes with 5-FU (0.5, 5, 10, 25 mg/ml), washed and further incubated for 24 hr. Optical density (OD) of the fibroblasts was measured using MTT assay. Growth rates (%) were calculated as [100 × (OD values of drug-treat-ed cells/OD values of non-treated cells)]. Values and bars represent means of growth rates and the SD, respectively. ∗ p<0.05 compared to non-treated control. 5-FU = 5-fluorouracil. |
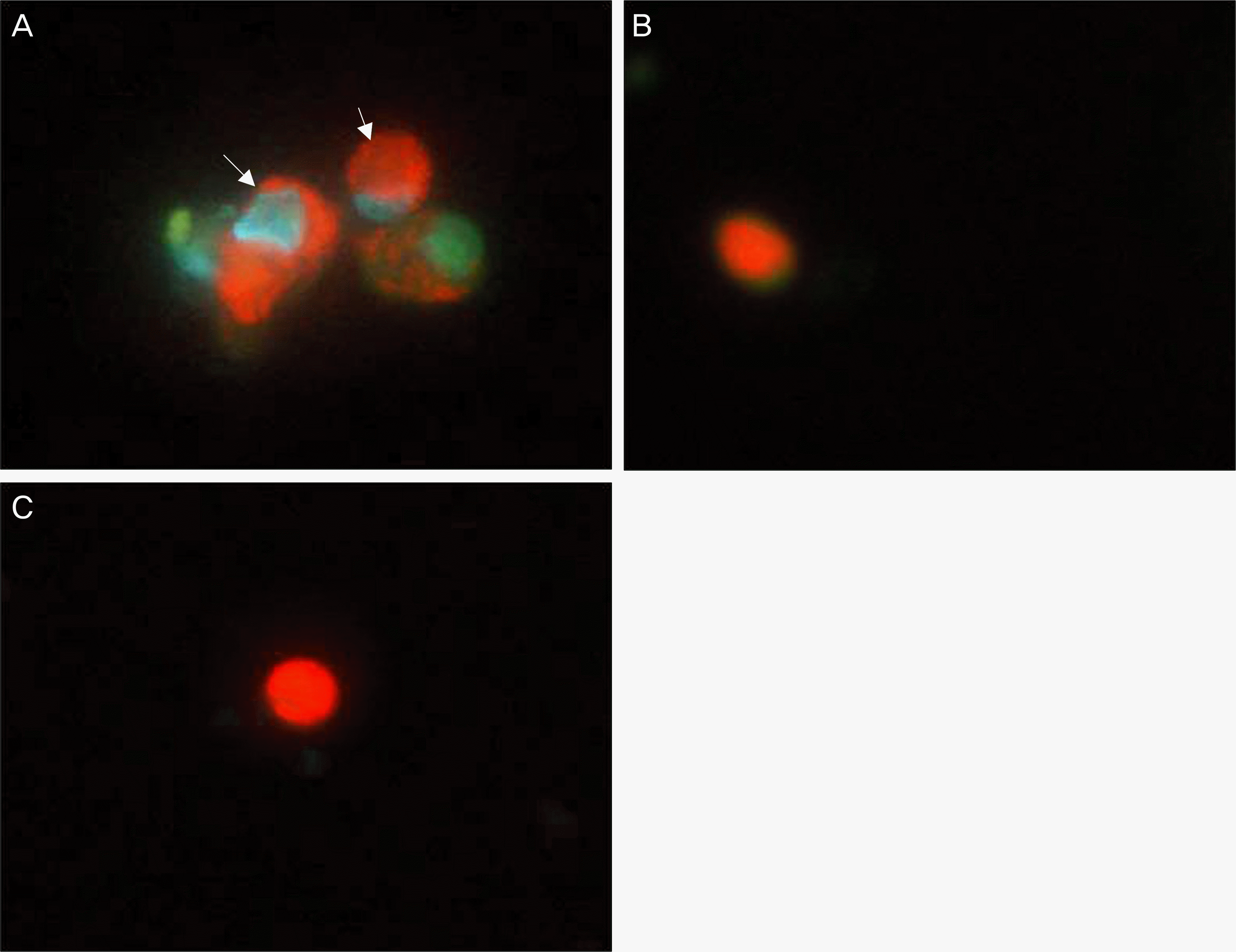 | Figure 5.Acridine orange/Hoechst (AO/HO) staining of human nasal mucosa fibroblasts after treatment with MMC and 5-FU. Fibroblasts were not-treated (A), or treated with 0.4 mg/ml of MMC (B) and 25 mg/ml of 5-FU (C) for 5 minutes, washed and further incubated for 3 hr. The cells were then stained with AO/HO to identify apoptosis as shown in the Methods. (A) Normal control cells. Arrow indicates blue nuclei and brilliant red cytoplasm. (B) MMC-treated cells. The apoptotic cell shows orange-red nuclei, becoming indistinguishable from the cytoplasm. (C) 5-FU-treated cells. The apoptotic cell shows orange-red nuclei, becoming indistinguishable from the cytoplasm. Magnification, ×400. MMC = mitomycin C, 5-FU = 5-fluorouracil. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download