Abstract
Purpose
To investigate the effects of erythropoietin (EPO) on the production of nitric oxide in cultured human trabecular meshwork cells (HTMC).
Methods
Primarily cultured HTMC were exposed to 0, 0.5, and 1.0 U/ml EPO using serum-deprived media for 2 days. Production of nitric oxide and cellular survival were assessed with Griess assay and MTT assay, respectively. Expression of EPO mRNA receptor and activity of eNOS were assessed with RT-PCR.
Go to : 
References
1. Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary openangle glaucoma and nonglaucomatous normals. Ophthalmology. 1984; 91:564–79.

2. Rohen JW, Lütjen-Drecoll E, Flügel C, et al. Ultrastructure of the trabecular meshwork in untreated cases of primary openangle glaucoma (POAG). Exp Eye Res. 1993; 56:683–92.
3. Wiederholt M, Dorschner N, Groth J. Effect of diuretics, channel modulators, and signal interceptors on contractility of the trabecular meshwork. Ophthalmologica. 1997; 211:153–60.

4. Wiederholt M, Stumpff F. The trabecular meshwork and aqueous humor reabsorption. Civan MM, editor. Current Topics in Membranes. The Eye's Aqueous Humor: From Secretion to Glaucoma. 45. San Diego: Academic Press;1998. p. 163–202.
5. Wiederholt M, Sturm A, Lepple-Wienhues A. Relaxation of trabecular meshwork and ciliary muscle by release of nitric oxide. Invest Ophthalmol Vis Sci. 1994; 35:2515–20.
6. Behar-Cohen FF, Goureau O, D'Hermies F, Courtois Y. Decreased intraocular pressure induced by nitric oxide donors is correlated to nitrite production in the rabbit eye. Invest Ophthalmol Vis Sci. 1996; 37:1711–5.
7. Fandrey J. Oxygen-dependent and tissue-specific regulation of erythropoietin gene expression. Am J Physiol Regul Integr Comp Physiol. 2004; 286:R977–88.

8. Siren AL, Fratelli M, Brines M, et al. Erythropoietin prevents neu-ronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA. 2001; 98:4044–9.

9. Carlini RG, Alonzo EJ, Dominguez J, et al. Effect of recombinant human erythropoietin on endothelial cell apoptosis. Kidney Int. 1999; 55:546–53.

10. Akimoto T, Kusano E, Inaba T, et al. Erythropoietin regulates vascular smooth muscle cell apoptosis by a phosphatidylinositol 3 kinase-dependent pathway. Kidney Int. 2000; 58:269–82.

11. Rui T, Feng Q, Lei M, et al. Erythropoietin prevents the acute my-ocardial inflammatory response induced by ischemia/reperfusion via induction of AP-1. Cardiovasc Res. 2005; 65:719–27.

12. Burger D, Lei M, Geoghegan-Morphet N, et al. Erythropoietin protects cardiomyocytes from apoptosis via up-regulation of endothelial nitric oxide synthase. Cardiovasc Res. 2006; 72:51–9.

13. Garcia-Ramirez M, Hernandez C, Simo R. Expression of Erythropoietin and Its Receptor in the Human Retina: comparative study of diabetic and nondiabetic subjects. Diabetes Care. 2008; 31:1189–94.
14. Tsai JC, Song BJ, Wu L, Forbes M. Erythropoietin: A candidate neuroprotective agent in the treatment of glaucoma. J Glaucoma. 2007; 16:567–71.

15. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.

16. Freimoser FM, Jakob CA, Aebi M, Tuor U. The MTT [3-(4,5-di-methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay is a fast and reliable method for colorimetric determination of fungal cell densities. Appl Environ Microbiol. 1999; 65:3727–9.

17. Green LC, Wagner DA, Glogowski J, et al. Analysis of Nitrate, Nitrite and [15N]Nitrate in biological fluids. Anal Biochem. 1982; 126:131–8.

18. Smith KJ, Bleyer AJ, Little WC, Sane DC. The cardiovascular effects of erythropoietin. Cardiovasc Res. 2003; 59:538–48.

19. Santhanam AV, Smith LA, Akiyama M, et al. Role of endothelial NO synthase phosphorylation in cerebrovascular protective effect of recombinant erythropoietin during subarachnoid hemorrhage-induced cerebral vasospasm. Stroke. 2005; 36:2731–7.
20. Urao N, Okigaki M, Yamada H, et al. Erythropoietin-mobilized endothelial progenitors enhance reendothelialization via Akt-endothelial nitric oxide synthase activation and prevent neointimal hyperplasia. Circ Res. 2006; 98:1405–13.

21. d'Uscio LV, Smith LA, Santhanam AV, et al. Essential role of endothelial nitric oxide synthase in vascular effects of erythropoietin. Hypertension. 2007; 49:1142–8.
22. d'Uscio LV, Katusic ZS. Erythropoietin increases endothelial bio-synthesis of tetrahydrobiopterin by activation of protein kinase B alpha/Akt1. Hypertension. 2008; 52:93–9.
23. d'Uscio LV, Smith LA, Katusic ZS. Erythropoietin increases expression and function of vascular copper- and zinc-containing su-peroxide dismutase. Hypertension. 2010; 55:998–1004.
24. Wang XQ, Vaziri ND. Erythropoietin depresses nitric oxide synthase expression by human endothelial cells. Hypertension. 1999; 33:894–9.

25. Mokbel TH, Ghanem AA, Kishk H, et al. Erythropoietin and soluble CD44 levels in patients with primary openangle glaucoma. Clin Experiment Ophthalmol. 2010; 38:560–5.

26. Kawakami M, Sekiguchi M, Sato K, et al. Erythropoietin re-ceptor-mediated inhibition of exocytotic glutamate release confers neuroprotection during chemical ischemia. J Biol Chem. 2001; 276:39469–75.

27. Arjamaa O, Nikinmaa M. Oxygen-dependent diseases in the retina: role of hypoxia-inducible factors. Exp Eye Res. 2006; 83:473–83.

Go to : 
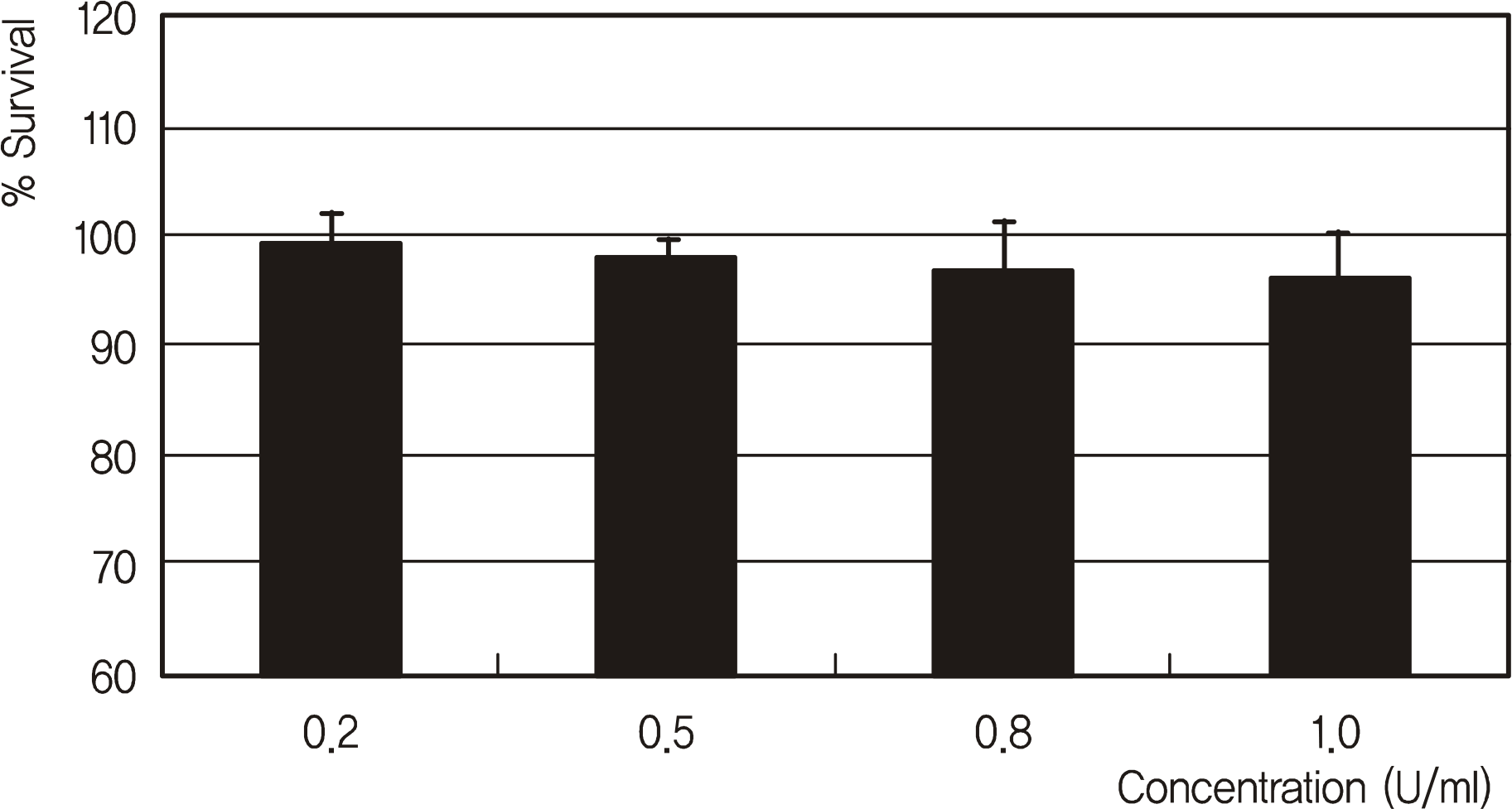 | Figure 1.Effects of erythropoietin on the survival of trabecular meshwork cells. Erythropoietin did not affect cellular survival (p > 0.05). |
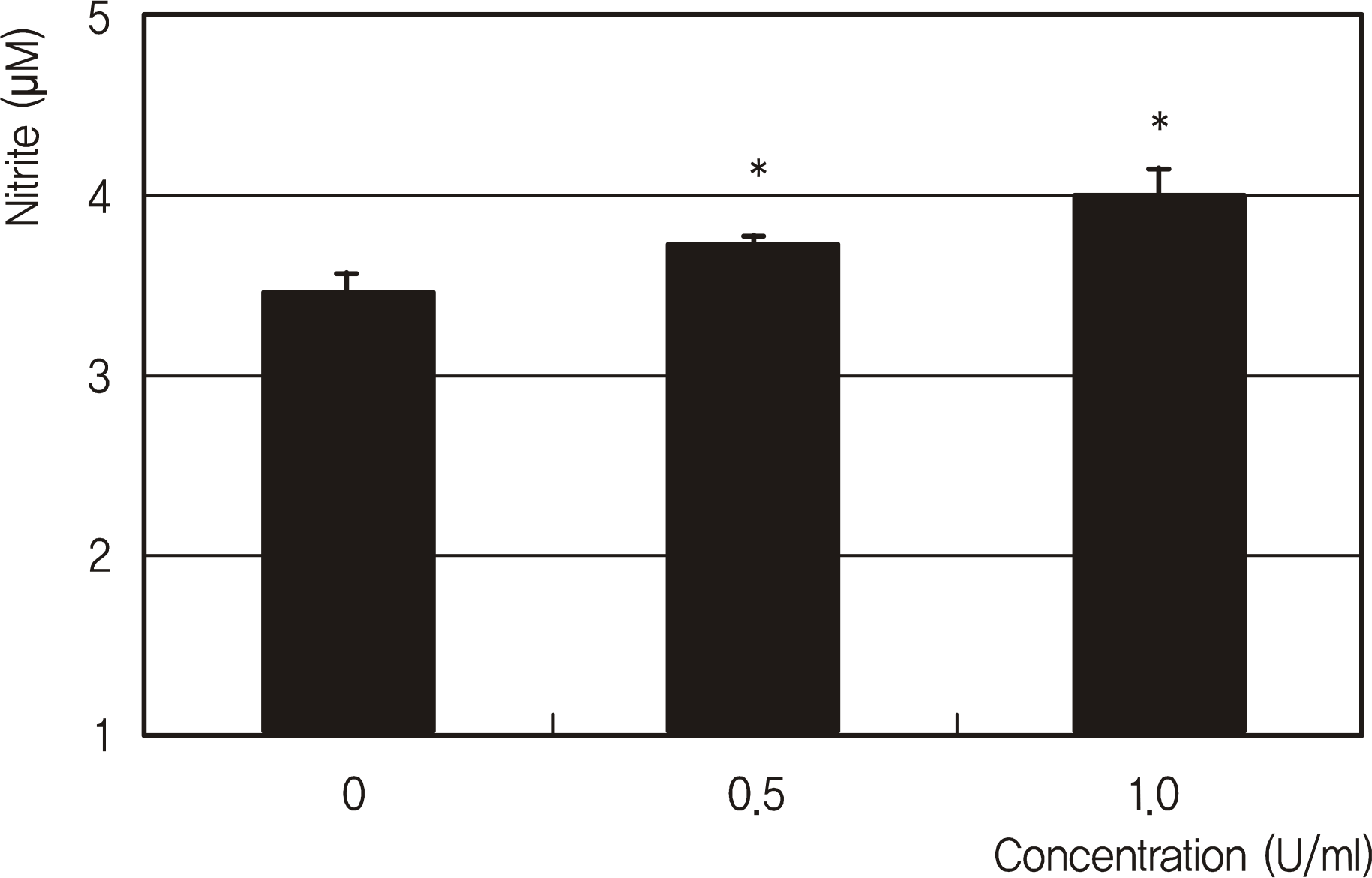 | Figure 2.Effects of erythropoietin on the production of nitric oxide in trabecular meshwork cells. Erythropoietin increased NO production (∗ p < 0.05). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download