Abstract
Purpose
To evaluate the outcomes of Ahmed valve implantation in neovascular glaucoma patients who received intraoperative mitomycin C (MMC) and postoperative 5-fluorouracil (5-FU) after 24 months of followup.
Methods
A total of 40 eyes from 40 patients with neovascular glaucoma who received antiglaucomatous medication without previous glaucoma surgery were included in the present study. The patients were divided into 2 groups. The control group (20 eyes) underwent Ahmed valve implantation only and the study group (20 eyes) underwent Ahmed valve implantation and received intraoperative MMC and postoperative 5-FU. Failure was defined as the first occurrence of any of the following: 1) the first of 3 consecutive visits where intraocular pressure (IOP) was over 18 mmHg; 2) 20% IOP reduction from baseline; 3) the final number of topical medications was not reduced by at least two from baseline; 4) the need for additional surgery; or 5) the occurrence of a serious complication.
References
1. Coleman AL, Hill R, Wilson MR, et al. Initial clinical experience with Ahmed glaucoma valve implant. Am J Ophthalmol. 1995; 120:23–31.
2. Allen RC, Bellow AR, Hutchinson BT. Filtration surgery in the treatment of neovascular glaucoma. Ophthalmology. 1982; 89:1181–7.

3. Sutton GE, Popp JC, Records RE. Krupin Denver valve and neovascular glaucoma. Trans Ophthalmol Soc U K. 1982; 102:119–21.
4. Lee SH, Ma KT, Hong YJ. Outcome of Ahmed valve implantation in refractory glaucoma. J Korean Ophthalmol Soc. 2007; 48:83–90.
5. Yoon HJ, Park JJ. Ahmed Valve Implantation with adjunctive mitomycin C and 5-fluorouracil: Outcomes at one year. J Korean Ophthalmol Soc. 2010; 51:227–33.

6. Guo W, Song Y, Sun X. Ahmed valve Implantation for refractory glaucoma. Zhonghua Yan Ke Za Zhi. 1997; 33:417–20.
7. Yalvac IS, Eksioglu U, Santana B, Duman S. Long term results of Ahmed glaucoma valve and Molteno implant in neovascular glaucoma. Eye. 2007; 21:65–70.
8. Im YW, Lym HS, Park CK, Moon JI. Comparison of mitomycin trabeculectomy and Ahmed valve implant surgery for neovascular glaucoma. J Korean Ophthalmol Soc. 2004; 45:1515–21.
9. Lee JJ, Park KH, Kim DM, Kim TW. Clinical outcomes of Ahmed glaucoma valve implantation using tube ligation and removable external stents. Korean J Ophthalmol. 2009; 23:86–92.

10. Son JY, Park SK, Kim YI. Histologic study after 5-fluorouracil injection into the bleb in Ahmed valve implanted rabbit eyes. J Korean Ophthalmol Soc. 2003; 44:2144–452.
11. Lee YW, Yim JH, Lee SB. The factors associated with the success of Ahmed glaucoma valve implantation. J Korean Ophthalmol Soc. 2005; 46:1509–17.
12. Skuta GL, Parrish RK. Wound healing in glaucoma filtering surgery. Surv Ophthalmol. 1987; 32:149–70.

13. Topouzis F, Coleman AL, Choplin N, et al. Followup of the original cohort with the Ahmed glaucoma valve implant. Am J Ophthalmol. 1999; 128:198–204.
14. Nouri-Mahdavi K, Caprioli J. Evaluation of the hypertensive phase after insertion of the Ahmed glaucoma valve. Am J Ophthalmol. 2003; 136:1001–8.

15. Susanna R, Nicolela MT, Takahashi WY. Mitomycin C as adjunctive therapy with glaucoma implant surgery. Ophthalmic Surg. 1994; 25:458–62.

16. Kook MS, Yoon J, Kim J, Lee MS. Clinical results of Ahmed glaucoma valve implantation in refractory glaucoma with adjunctive mitomycin C. Ophthalmic Surg Lasers. 2000; 31:100–6.

17. Kurnaz E, Kubaloglu A, Yilmaz Y, et al. The effect of adjunctive mitomycin C in Ahmed glaucoma valve implantation. Eur J Ophthalmol. 2005; 15:27–31.

18. Lee JH, Kim SS, Hong YJ. A clinical study of the Ahmed valve implant in refractory glaucoma. J Korean Ophthalmol Soc. 2001; 42:1003–10.
19. Ayyala RS, Zurakowski D, Smith JA, et al. A clinical study of the Ahmed glaucoma valve implant in advanced glaucoma. Ophthalmology. 1998; 105:1968–76.
20. Abraham LM, Selva D, Casson R, Leibovitch I. The clinical applications of fluorouracil in ophthalmic practice. Drugs. 2007; 67:237–55.

21. Woo KJ, Hyung SM. Effect of need revision of failed filtering blebs with different concentrations of mitomycin C. J Korean Ophthalmol Soc. 2008; 49:951–7.
22. Zilelioğ lu G, Uğ urbaş SH, Anadolu Y, et al. Adjunctive use of mitomycin C on endoscopic lacrimal surgery. Br J Ophthalmol. 1998; 82:63–6.
23. Gilman AG, Goodman LS, Gilman A. The Pharmacological Basis of Therapeutics. 6th ed.New York: Macmillan Publishing Co.;1980. p. 1295–300.
Figure 1.
Intraocular pressure (IOP) following Ahmed valve implantation only (control group) and Ahmed valve implantation with adjunctive intraoperative mitomycin C and postoperative 5-fluorouracil (study group). D = day; W = week; M = month.

Figure 2.
Kaplan-Meier estimates of the cumulative probability of valve success for Ahmed valve implanted eyes. Control group (20 eyes) received Ahmed valve implantation only. Study group (20 eyes) received Ahmed valve implantation and intraoperative MMC and postoperative 5-FU. Failure was defined as the first occurrence following an initial postoperative period of any of the following events: 1) IOP > 18 mm Hg for three consecutive visits or <20% IOP reduction from baseline and the final number of topical medications needed to be not less than at least 2 from baseline, 2) need for additional surgery to repair a malfunctioning Ahmed valve, or 3) serious postoperative complications.
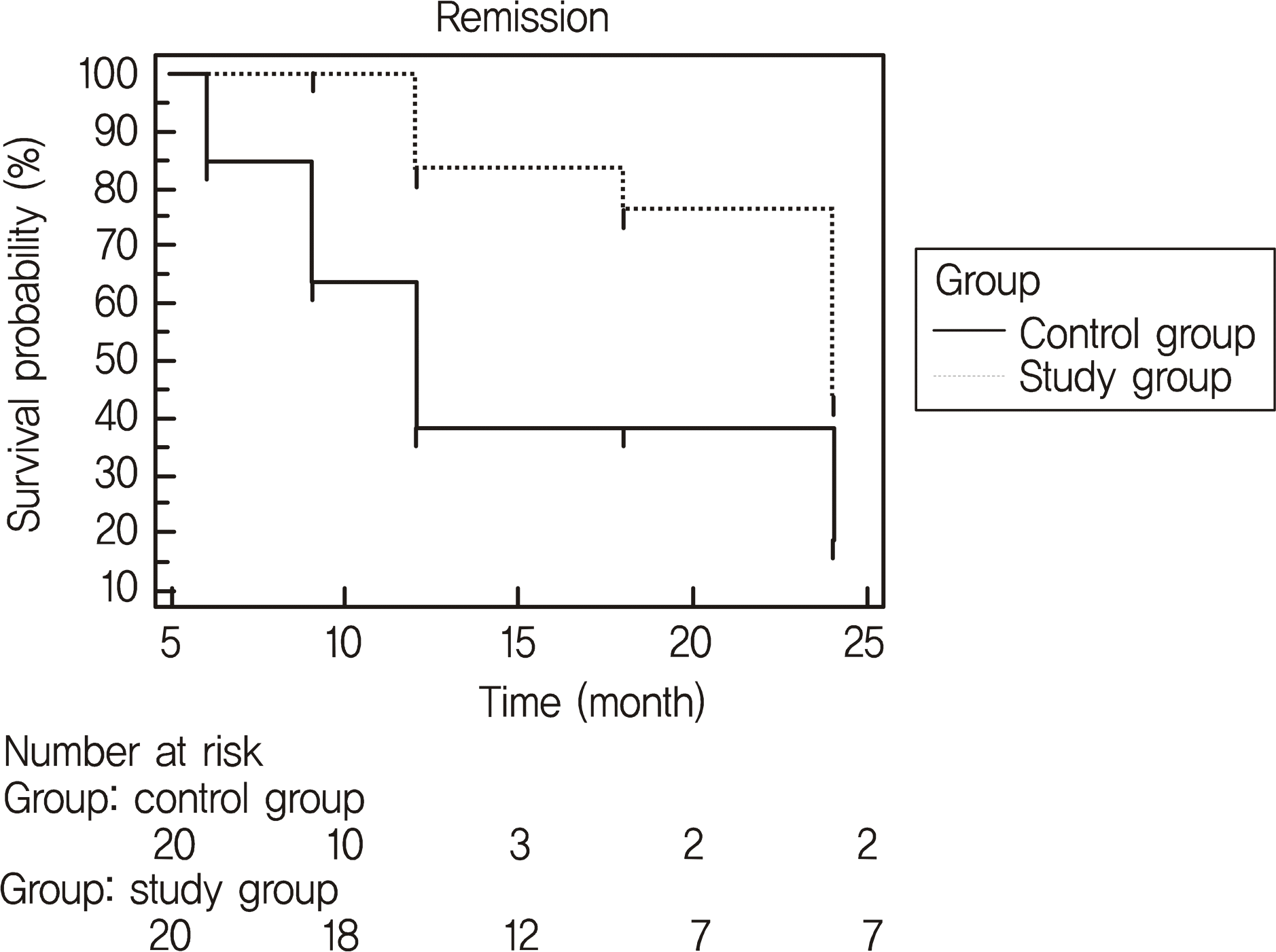
Table 1.
Baseline characteristics for Ahmed valve-implanted eyes
| Control group (n = 20) | Study group (n = 20) | p-value∗ | |
|---|---|---|---|
| Age (yr) | 57.80 ± 7.68 | 51.90 ± 10.40 | 0.08 |
| Sex | 0.821 | ||
| Male | 10 (50.0) | 11 (55.0) | |
| Female | 10 (50.0) | 9 (45.0) | |
| Diagnosis | 0.598 | ||
| NVG with DMR | 17 (85) | 18 (90) | |
| NVG with CRVO | 1 (5) | 0 (0) | |
| NVG with OIS | 2 (10) | 2 (10) | |
| Lens status | 0.946 | ||
| Aphakic | 2 (10.0) | 2 (10.0) | |
| Phakic | 8 (40.0) | 9 (45.0) | |
| Pseudophakic | 10 (50.0) | 9 (45.0) | |
| Preoperative IOP (mm Hg) | 38.50 ± 5.31 | 39.10 ± 5.88 | 0.731 |
| No. of preoperative-medication | 3.05 ± 0.60 | 2.85 ± 0.81 | 0.428 |
| MMC time (min) | 0 | 4.72 ± 0.55 | |
| No. of 5-FU injections | 0 | 3.27 ± 0.45 | |
| Follow up period (mon) | 26.8 ± 4.72 | 28.2 ± 5.22 | 0.94 |
Table 2.
Postoperative intraocular pressure for Ahmed valve implanted eyes during the followup period
| Time | IOP of control group (mm Hg) | IOP of study group (mm Hg) | p-value∗ |
|---|---|---|---|
| Baseline | 38.50 ± 5.31 | 39.10 ± 5.88 | 0.731 |
| 1 day | 13.70 ± 2.47 | 12.85 ± 2.87 | 0.281 |
| 1 wk | 13.35 ± 1.39 | 13.50 ± 3.10 | 0.821 |
| 1 mon | 14.65 ± 1.42 | 13.65 ± 2.01 | 0.116 |
| 3 mon | 15.30 ± 1.75 | 14.35 ± 2.21 | 0.198 |
| 6 mon | 16.60 ± 2.58 | 15.15 ± 3.90 | 0.200 |
| 9 mon | 18.55 ± 3.03 | 15.30 ± 5.11 | 0.047 |
| 12 mon | 18.90 ± 3.86 | 14.20 ± 5.75 | 0.011 |
| 18 mon | 19.55 ± 3.90 | 14.75 ± 5.18 | 0.005 |
| 24 mon | 19.95 ± 3.93 | 14.80 ± 4.76 | 0.002 |
Table 3.
Kaplan-Meier estimates of probability of success in Ahmed valve implanted eyes
Table 4.
Postoperative characteristics of control and study group
| Control group | Study group | p-value∗ | |
|---|---|---|---|
| Hypertensive phase: | |||
| Yes | 7.00 (35) | 4.00 (20) | 0.048 |
| Postoperative no. of medications at 24 months | 1.40 ± 0.94 | 0.55 ± 0.69 | 0.022 |
| Change in no. of medication from baseline | ‐1.65 ± 0.81 | ‐2.30 ± 0.92 | 0.049 |
| Medications relative to baseline | |||
| Lower no. of medications | 15 (75) | 18 (90) | 0.081 |
| Same no. of medications | 5 (25) | 2 (10) | 0.068 |
| Greater no. of medications | 0 | 0 |
Table 5.
Postoperative complications
| Control group | Study group | p-value∗ | |
|---|---|---|---|
| Transient hypotony | 2 (10) | 5 (25) | 0.032 |
| Transient hyphema | 3 (15) | 2 (10) | 0.245 |
| Retration of tube from anterior chamber | 2 (10) | 1 (5) | 0.435 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download