Abstract
Methods
Refraction, accommodation amplitude, residual accommodation and biometric findings were assessed before and after instillation of regimen I (tropicamide 0.5% and phenylephrine 0.5%), regimen II (cyclopentolate 1.0%), and regimen III (combination of regimen I and II).
Results
In myopic adults aged 22 to 26 years, cycloplegic refraction revealed less myopia than manifested refraction. Although there was no difference in residual accommodation among the 3 regimens, regimen II and III were more effective in reducing myopia, accommodation, and axial length. The difference in cycloplegic refraction between regimen I and II was more prominent in patients who had larger amplitude of accommodation and residual accommodation with regimen I.
References
1. Moon NJ, Kim JC, Koo BS. The study on the necessity of cycloplegic refraction in school children. J Korean Ophthalmol Soc. 1988; 29:377–85.
2. Kim CK, Hong SH. Changes in refractive finding after using cycloplegics in young adult. J Korean Ophthalmol Soc. 1984; 25:341–5.
3. Bannon RE. The use of cycloplegics in refraction. Am J Optom Arch Am Acad Optom. 1947; 24:513–68.

4. Maoury SD. Comparison of fixation targets during noncycloplegic retinoscopy. Am J Ophthalmol. 1967; 63:865.

5. Hiatt RL, Braswell R, Smith L, Patty JW. Refraction using mydriatic, cycloplegic, and manifest techniques. Am J Ophthalmol. 1973; 76:739–44.

6. Hofmeister EM, Kaupp SE, Schallhorn SC. Comparison of tropicamide and cyclopentolate for cycloplegic refractions in myopic adult refractive surgery patients. J Cataract Refract Surg. 2005; 31:694–700.

7. Yang SW, Lee NY, Kim SY. The effect of cycloplegia on vision and stereopsis: comparison between before and after cycloplegia. J Korean Ophthalmol Soc. 2006; 47:1454–8.
8. Rosenfield M, Cohen AS. Repeatability of clinical measurements of the amplitude of accommodation. Ophthalmic Physiol Opt. 1996; 16:247–9.

9. Atchison DA, Capper EJ, McCabe KL. Critical subjective measurements of amplitude of accommodation. Optom Vis Sci. 1994; 71:699–706.
10. Gettes BC, Leopold IH. Evaluation of five new cycloplegic drugs. AMA Arch Ophthalmol. 1953; 49:24–7.

11. Lancaster WZ, Williams ER. New light on the theory of accommodation with practical application. Trans Am Acad Ophthalmol Otolaryngol. 1914; 19:170–95.
12. Ciuffreda KJ. Near work- induced transient myopia: basic and clinical aspects. J Optom Vis Dev. 1999; 30:5–20.
13. Ciuffreda KJ, Wallis DM. Myopes show increased susceptibility to nearwork aftereffects. Invest Ophthalmol Vis Sci. 1998; 39:1797–803.
14. Ciuffreda KJ, Lee M. Differential refractive susceptibility to sus-tained nearwork. Ophthalmic Physiol Opt. 2002; 22:372–9.

15. Wolffsohn JS, Gilmartin B, Thomas R, Mallen EA. Refractive error, cognitive demand and nearwork-induced transient myopia. Curr Eye Res. 2003; 27:363–70.

16. Duane A. Studies in monocular and binocular accommodation with their clinical applications. Trans Am Ophthalmol Soc. 1922; 20:132–57.

17. Priestley BS, Medine MM. A new mydriatics and cycloplegic drug. Compound 75 G. T. Am J Ophthalmol. 1951; 34:572–5.
18. Gordon DM, Ehrenberg MH. Cyclopentolate hydrochloride: a new mydriatic and cycloplegic agent; a pharmacologic and clinical evaluation. Am J Ophthalmol. 1954; 38:831–8.
19. Miranda MN. Residual accommodation. A comparison between cyclopentolate 1 percent and a combination of cyclopentolate 1 percent and tropicamaide 1 percent. Arch Ophthalmol. 1972; 87:515–7.
20. Stine GT. Clinical investigation of a new cycloplegic and mydriatic drug. Eye Ear Nose Throat Mon. 1960; 22:11–4.
22. Choi O, Choo CH, Cho SC. The studies on the residual accommodation of Koreans. II. The residual accommodation under 0.5 percent scopolamine and 1 percent cyclogyl cycloplegia. Yonsei Med J. 1964; 5:62–4.
23. Ebri A, Kuper H, Wedner S. Cost-effectiveness of cycloplegic agents: results of a randomized controlled trial in Nigerian children. Invest Ophthalmol Vis Sci. 2007; 48:1025–31.

24. Choi YH, Choi YY. The difference comparison according to child refractive method and effect of life style on myopia. J Korean Ophthalmol Soc. 2005; 46:1841–7.
25. Gao L, Zhuo X, Kwok AK, et al. The change in ocular refractive components after cycloplegia in children. Jpn J Ophthalmol. 2002; 46:293–8.

26. McBrien NA, Moghaddam HO, Reeder AP. Atropine reduces experimental myopia and eye enlargement via a nonaccommodative mechanism. Invest Ophthalmol Vis Sci. 1993; 34:205–15.
27. Luft WA, Ming Y, Stell WK. Variable effects of previously un-tested muscarinic receptor antagonists on experimental myopia. Invest Ophthalmol Vis Sci. 2003; 44:1330–8.

28. Gwiazda J, Marsh-Tootle WL, Hyman L, et al. Baseline refractive and ocular component measures of children enrolled in the correction of myopia evaluation trial (COMET). Invest Ophthalmol Vis Sci. 2002; 43:314–21.
29. Saw SM, Chua WH, Hong CY, et al. Nearwork in early-onset myopia. Invest Ophthalmol Vis Sci. 2002; 43:332–9.
30. Kim JC, Koo BS. A study of prevailing features and causes of myopia and visual impairment in urban school children. J Korean Ophthalmol Soc. 1988; 29:165–81.
Figure 1.
Comparison in changes of refractive errors following instillation of regimen I, Regimen II, and Regimen III. Regimen I: tropicamide 0.5% and phenylephrine HCl 0.5%; Regimen II: cyclopentolate 1.0%; Regimen III: combination of regimen I and regimen II. ∗The difference between the two regimens was statistically significant. Error bars, 0.95 CI.
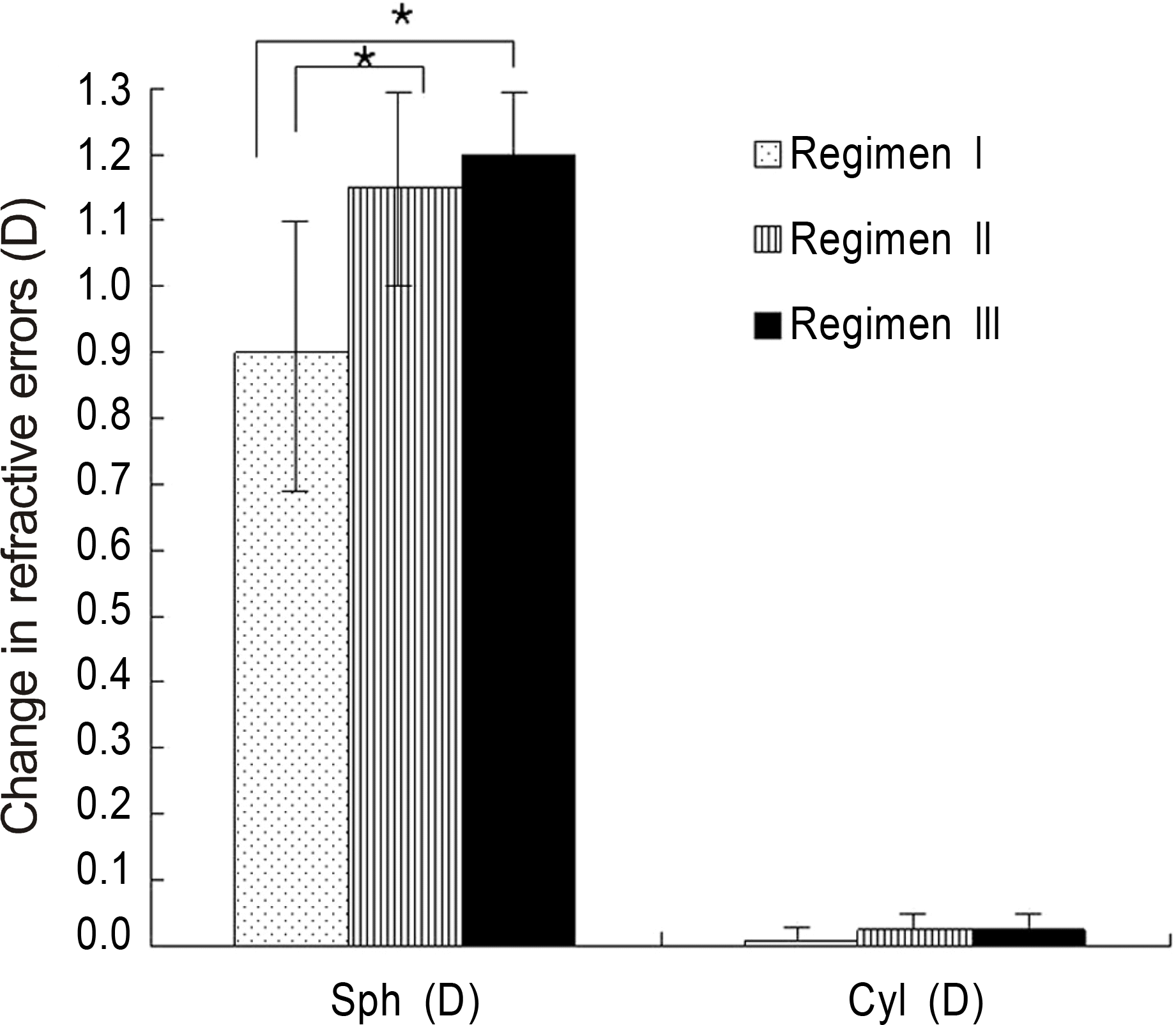
Figure 2.
Comparison in changes of refractive errors following cycloplegia in respect with amount of refractive errors. Regimen I: tropicamide 0.5% and phenylephrine HCl 0.5%; Regimen II: cyclopentolate 1.0%; Regimen III: combination of regimen I and regimen II. ∗The difference between the two regimens was statistically significant. Error bars, 0.95 CI.
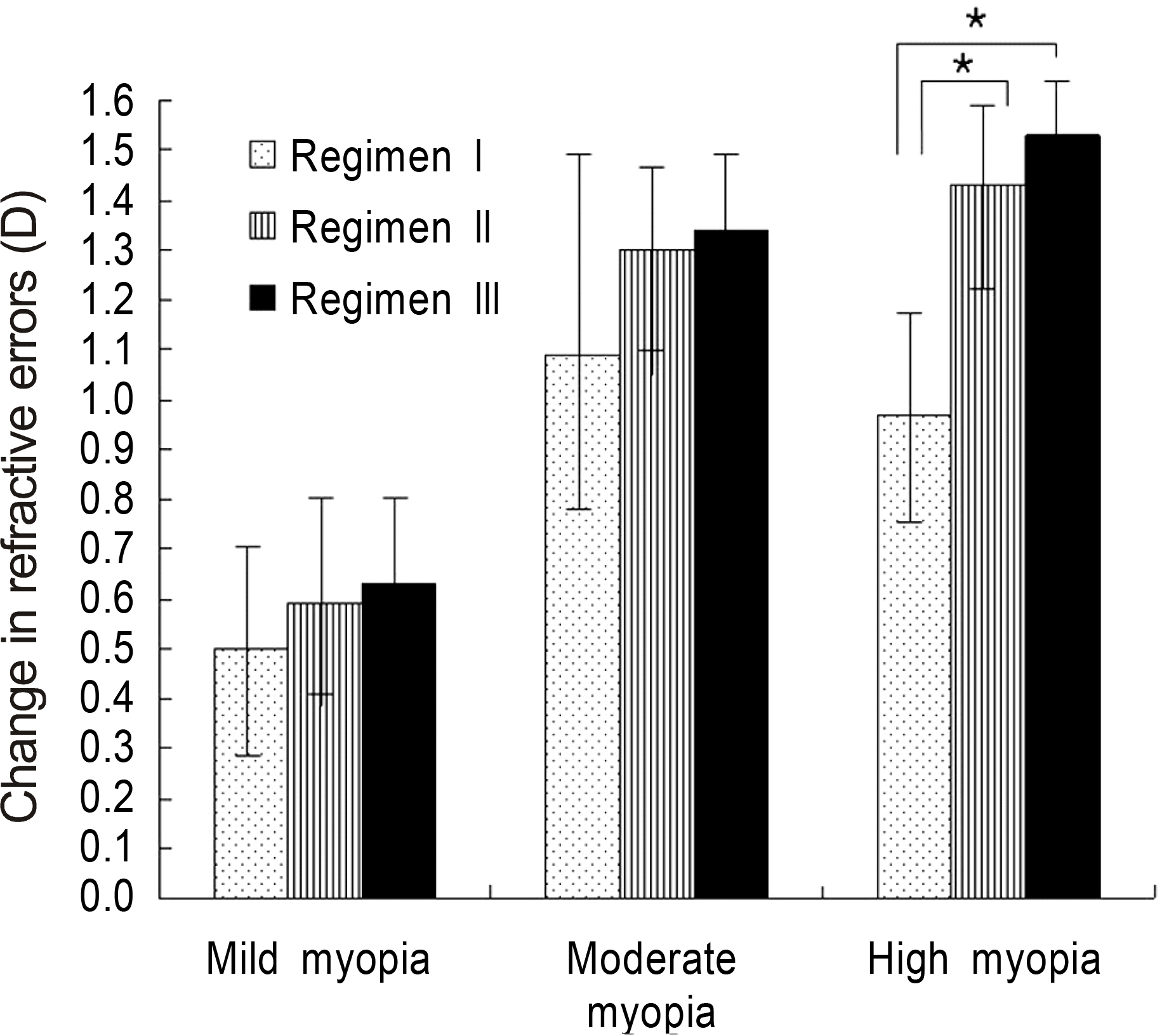
Table 1.
Comparison of effects of three regimens on accommodation amplitude and residual accommodation
| No instillation | Regimen I | Regimen II | Regimen III | p-value | |
|---|---|---|---|---|---|
| Accommodation amplitude measured by NPA∗ | 11.73 ± 3.27 | 4.94 ± 2.17 | 3.43 ± 1.60 | 3.33 ± 1.62 | 0.001 |
| Mild myopia (n = 8) | 9.46 ± 1.04 | 3.80 ± 1.28 | 3.37 ± 1.39 | 3.32 ± 1.19 | 0.885 |
| Moderate myopia (n = 14) | 12.58 ± 3.76 | 5.08 ± 2.25 | 3.38 ± 1.53 | 3.37 ± 1.40 | 0.018 |
| High myopia (n = 8) | 12.51 ± 2.98 | 5.81 ± 2.48 | 3.59 ± 0.70 | 3.30 ± 0.86 | 0.011 |
| Residual accommodation | NA† | 1.96 ± 1.21 | 0.73 ± 0.56 | 0.73 ± 0.47 | 0.212 |
| Mild myopia (n = 8) | NA | 2.23 ± 1.19 | 1.37 ± 0.37 | 1.37 ± 0.04 | 0.928 |
| Moderate myopia (n = 14) | NA | 1.83 ± 1.29 | 0.54 ± 0.32 | 0.52 ± 0.36 | 0.067 |
| High myopia (n = 8) | NA | 1.31 ± 1.10 | 0.29 ± 0.06 | 0.30 ± 0.28 | 0.366 |
Table 2.
Comparison of effects of three regimens on pupil size and biometric findings using ultrasound measurement
Table 3.
The clinical factors which could affect differences in refractive changes between regimen I (tropicamide 0.5% and phenylephrine HCl 0.5%) and regimen II (cyclopentolate 1.0%)
| Correlation coefficients∗ | p-value | |
|---|---|---|
| Spherical equivalent on no instillation | 0.224 | 0.234 |
| Axial length on no instillation | 0.150 | 0.430 |
| Accommodation amplitude on no instillation | 0.373 | 0.042 |
| Residual accommodation on instillation of regimen | 0.597 | 0.001 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download