Abstract
Purpose
To evaluate the surgical results and complications arising from scleral graft and free conjunctival autograft using tissue adhesive and temporary amniotic membrane transplantation as the surgical treatment for scleromalacia.
Methods
Scleral graft and free conjunctival autograft using tissue adhesive and temporary amniotic membrane transplantation was performed in 20 eyes of 20 patients with scleromalacia caused by pterygium excision. The surgical results and complications arising from the procedure were monitored and analyzed through followup.
Results
During the followup period of 17.6 ± 5.9 months, the wound injection and edema at the free conjunctival autograft and operation site healed in all the cases except 1 at postoperative 1 month. The stability of the ocular surface for graft transplantation was maintained at postoperative 3 months. Although a partial absorption of the conjunctival autograft induced by careless treatment occurred 2 weeks postoperative in 1 case, the ocular surface stabilized due to suitable treatment after 6 months. Although the edges of the scleral graft in 3 patients were partially absorbed 6 months postoperatively, the ocular surface stability was maintained by covering the conjunctival autografts.
Go to : 
References
1. Hong SB, Oh SJ, Oh JH. The effects and complications of mitomycin C for prevention of recurrence after pterygium operation. J Korean Ophthalmol Soc. 1998; 39:2013–8.
2. Moriarty AP, Crawford GJ, Mcallister IL, Constable IJ. Severe cor-neoscleral infection. A complication of beta irradiation scleral necrosis following pterygium excision. Arch Ophthalmol. 1993; 111:947–51.
3. Kim JH, Lee HB, Yoon DK. Scleral grafts for the cases of scleral perforation, scleral ectasia and scleral necrosis. J Korean Ophthalmol Soc. 1978; 19:55–61.
4. Kim JH. Scleral grafting on necrotic scleritis following pterygium excision. J Korean Ophthalmol Soc. 1982; 23:29–39.
5. Rhee HS, Kim MS, Kim JH. Scleral graft on necrotic scleritis following pterygium excision. J Korean Ophthalmol Soc. 1987; 28:565–8.
6. Kim SY, Chung WS, Hahn DK. Surgical management of scleral necrosis. J Korean Ophthalmol Soc. 1995; 36:7–12.
7. Oh JH, Kim JC. Repair of scleromalacia with preserved scleral and amniotic membrane transplantation. J Korean Ophthalmol Soc. 2001; 42:810–6.
8. Na YS, Joo MJ, Kim JH. Results of scleral allografting on scleral necrosis following pterygium excision. J Korean Ophthalmol Soc. 2005; 46:402–9.
9. Sangwan VS, Jain V, Gupta P. Structural and functional outcome of sclera patch graft. Eye. 2007; 21:930–5.
10. Lin CP, Tsai MC, Wu YH, Shih MH. Repair of a giant scleral ulcer with preserved sclera and tissue adhesive. Ophthalmic Surg Lasers. 1996; 27:995–9.

11. Lee BH, Mun HJ, Park YJ, Lee KW. Scleral allografting and amniotic membrane transplantation with fibrin glue in the management of scleromalacia. J Korean Ophthalmol Soc. 2010; 51:485–91.

12. Torchia RT, Dunn RE, Pease PJ. Fascia lata grafting in scleromalacia perforans with lamellar corneal-scleral dissection. Am J Ophthalmol. 1968; 66:705–9.
13. Ahn BH, Seong KH, Lee EJ. The use of a temporal muscle fascia in the treatment of scleral defect. J Korean Ophthalmol Soc. 1983; 24:785–9.
14. Shon TS, Kim HM, Jung HR. Temporalis fascia grafting in scleral necrosis after pterygium excision. J Korean Ophthalmol Soc. 1992; 33:1054–9.
15. Sohn YH, Yu SL, Uhm KB. Surgical treatment of scleral ulceration as a complication of pterygium excision. J Korean Ophthalmol Soc. 1995; 36:1323–30.
16. Kwak JY, Chang HG. Autogenous temporalis fascia grafting and conjunctival flap transposition in scleromalacia after pterygium excision. J Korean Ophthalmol Soc. 2004; 45:180–6.
17. Lee CO, Jong SH, Lee JJ. Autologous simple conjunctival graft and conjunctiva/tenon graft on focal scleromalacia. J Korean Ophthalmol Soc. 1997; 38:1737–41.
18. Breslin CW, Katz JI, Kaufman HE. Surgical management of ne-crotizing scleritis. Arch Ophthalmol. 1977; 95:2038–40.

19. Mauriello JA Jr, Fiore PM, Pokorny KS, Cinotti DJ. Use of split-thickness dermal graft in the surgical treatment of corneal and scleral defects. Am J Ophthalmol. 1988; 105:244–7.

20. Song HY, Im JS, Kwak JY. Acellular dermal allograft transplantation in patients with scleromalacia after pterygium excision. J Korean Ophthalmol Soc. 2008; 49:1685–9.

21. Uy HS, Reyes JM, Flores JD, Lim-Bon-Siong R. Comparison of fibrin glue and sutures for attaching conjunctival autografts after pterygium excision. Ophthalmology. 2005; 112:667–71.

22. Yoon KC, Heo H, Jeong IY. The use of fibrin glue for conjunctival autotransplantation in pterygium. J Korean Ophthalmol Soc. 2006; 47:198–204.
23. Kim HH, Mun HJ, Park YJ, et al. Conjuctivolimbal autograft using a fibrin adhesive in pterygium surgery. Korean J Ophthalmol. 2008; 22:147–54.
24. Tseng SC, Prabhasawat P, Lee SH. Amniotic membrane transplantation for conjunctival surface reconstruction. Am J Ophthalmol. 1997; 124:765–74.

25. Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995; 14:473–84.

26. Shimazaki J, Yang HY, Tsubota K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology. 1997; 104:2068–76.

27. Kim JC, Tseng SC. The effects on inhibition of corneal neovascularization after human amniotic membrane transplantation in severely damaged rabbit corneas. Korean J Ophthalmol. 1995; 9:32–46.

28. Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999; 179:325–35.
30. Helveston EM, Wilson DL. A suture reinforced scleral sling, technique for suspension of the ptotic upper lid. Arch Ophthalmol. 1975; 93:643–5.
Go to : 
 | Figure 1.Surgical procedures of scleral graft, free conjunctival autograft using tissue adhesive (Tissucol Duo Quick®, Baxter AG, Vienna, Austria) and temporary amniotic membrane patching in scleromalacia. (A-D) After debridement of the necrotic and surrounding sclera tissue, a scleral graft of appropriate size was prepared. Fibrin tissue adhesive, Tissucol Duo Quick® was used to at-tach the precisely cut-to-fit scleral graft to the recipient site. (E-G) A free conjunctival autograft from the superior palpebral conjunctiva was prepared making sure it was a little larger than the scleral defect size. The conjunctival autograft and the recipient site were attached in the following manner: Tissucol Duo Quick® was applied to the recipient site and the conjunctival autograft was pressed firmly on to it making sure all the edges were matched with no conjunctival surfaces exposed. Then, the recipient site and conjunctival autograft were sutured with 10-0 nylon at 4 points. (H-I) A temporary amniotic membrane was sutured on top of the conjunctival autograft to protect and facilitate the healing of the implanted scleral graft and conjunctival autograft. It was removed 5 to 7 days after the operation. |
 | Figure 2.Photographs of patients undergoing free conjunctival autograft with scleral graft for restoration of scleromalacia. (A, B, C-1) Preoperative photographs show scleral thinning with exposed uveal tissue at the previous pterygium excision site. (A, B, C-2) In all cases, scleral grafts were completely covered by conjunctival autograft and injections and edemas of free conjunctival autografts decreased in the 3 month postoperative photographs. (A, B, C-3) Last followup photographs showed stable ocular surface and absence of irregular fibrovascular proliferations. |
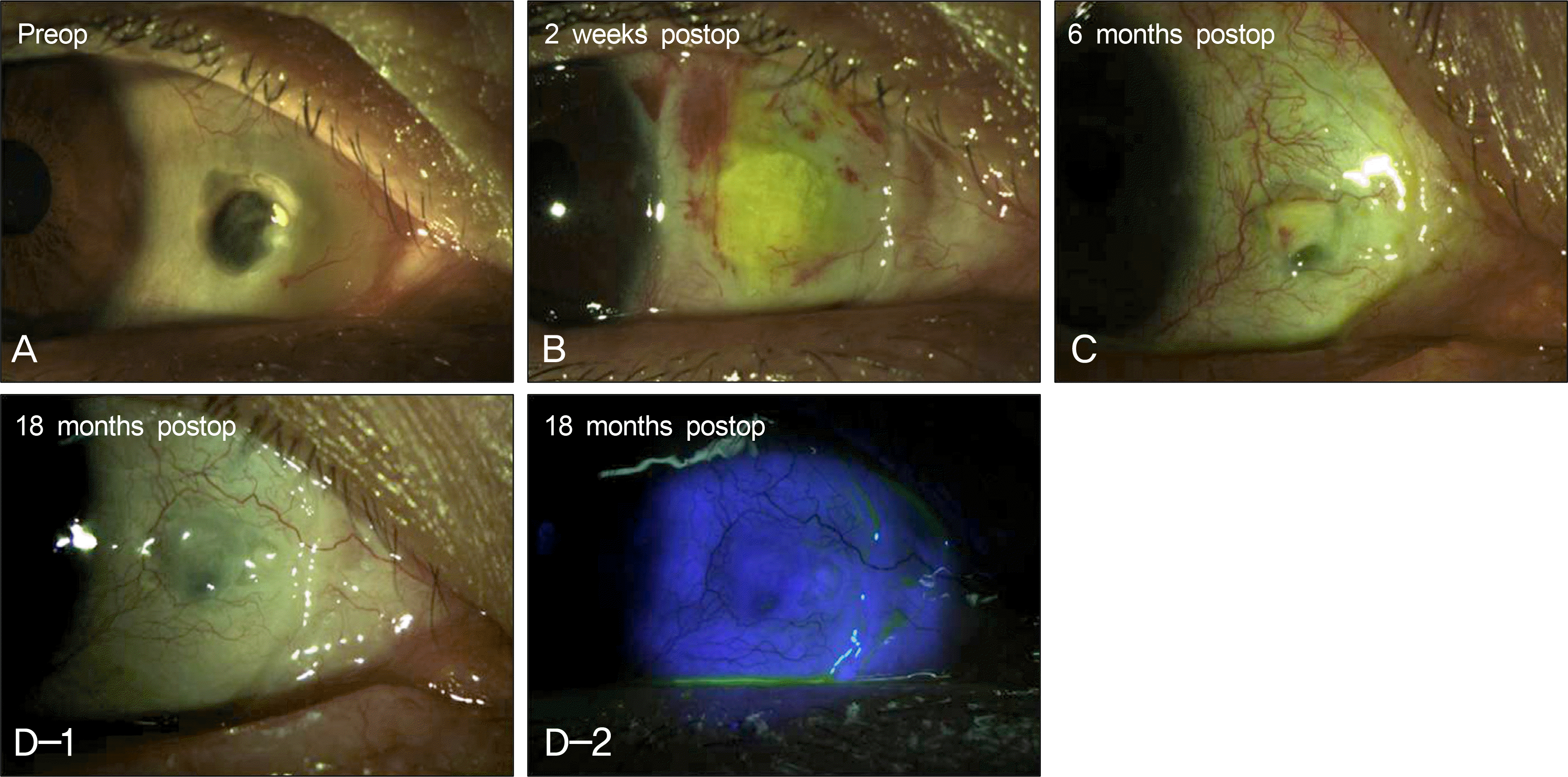 | Figure 3.A case photograph of scleromalacia in a 55 years old female after free conjunctival autograft with sclera graft. (A) Preoperative photograph showed sclera thinning with exposed uveal tissue and a severe avascular sclera bed at the site of previous pterygium excision. (B) Postoperative photograph at 2 weeks showed partial melting of the free conjunctival autograft due to steroid eye drop use following the operation (C) Postoperative photograph at 6 months showed partial absorption of the sclera graft and conjunctival autograft. (D-1, 2) Last followup photographs (18 months postop) showed relatively stable ocular surface with vascularized scleral bed and reepithelialization of the conjunctival autograft despite the additional absorption of the sclera graft. |
 | Figure 4.Photographs of scleral graft edge partial absorption after free conjuntival autograft with scleral graft. (A, B-1) Preoperative photographs show scleral thinning with exposed uveal tissue at the sites of previous pterygium excision. (A, B-2) Scleral grafts were completely covered by free conjunctival autografts and edemas of conjunctival autografts decreased in postoperative photographs at 3 months. (A, B-3) Stable ocular surfaces, with complete coverage of conjunctival autograft were shown despite the partial absorptions of the scleral grafts edges (black arrow) in postoperative 6 to 12 months photographs. |
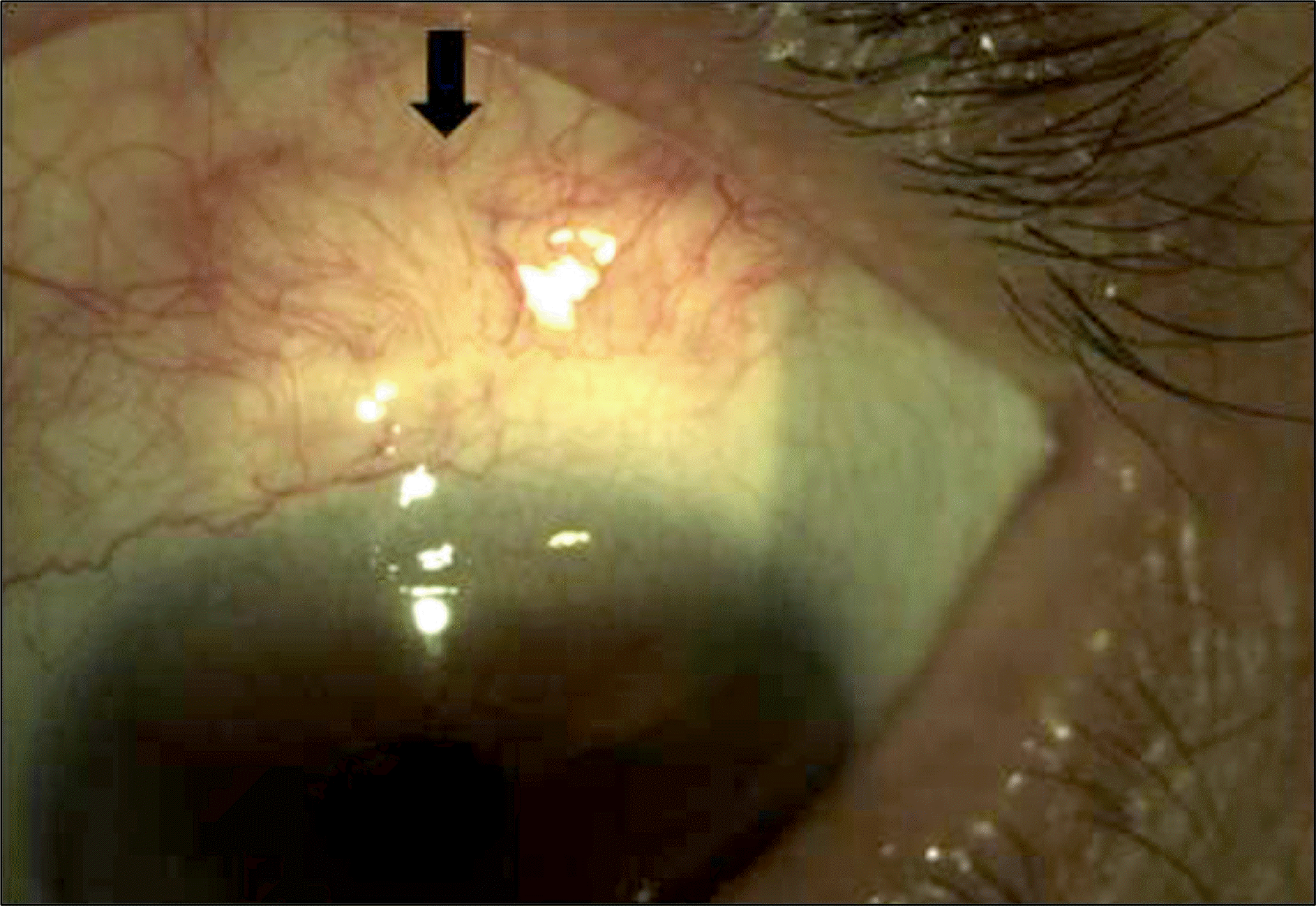 | Figure 5.Excessive vascularization (black arrow) at donor site in free conjunctival autograft with scleral graft for restoration of scleromalacia. |
Table 1.
Clinical characteristics, outcomes and complications of patients undergoing free conjunctival autograft with scleral graft for repair of scleromalacia




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download