Abstract
Purpose
The present study investigated whether an autophagic process is involved in the apoptotic death of human tenon's capsule fibroblasts (HTCFs) caused by mitomycin-C.
Methods
An autophagic phenotype was tested using fluorescence microscopy and flow cytometry with specific biological staining dyes including monodansylcadaverine and acridine orange and microtubule-associated protein 1 light chain 3 (LC3).
Results
Treatment with mitomycin-C (0.4 mg/ml) increased the acidic vesicular organelles of tenon's capsule fibroblasts in a time dependent manner. Mitomycin-C induced both LC3-II cleavage and beclin-1 expression. 3-MA, a pharmacological inhibitor of autophagy, inhibited the mitomycin-C induced increase of acidic vesicular organelleS.
Go to : 
References
2. Levine B, Klionsky DJ. Development by self-digestion molecular mechanisms and biological functions of autophagy. Dev Cell. 2004; 6:463–77.
3. Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004; 306:990–5.

4. Edinger AL, Thompson CB. Death by design: apoptosis, necrosis, and autophagy. Curr Opin Cell Biol. 2004; 16:663–9.

5. Beckers HJ, Kinders KC, Webers CA. Five-year results of trabeculectomy with mitomycin C. Graefes Arch Clin Exp Ophthalmol. 2003; 241:106–10.

6. Oh JH, Kim HK. The Effect of Preoperative Subconjunctival Injection of Mitomycin C and Triamcinolone in Recurrent Pterygium. J Korean Ophthalmol Soc. 2009; 50:1005–14.

7. Seong GJ, Park C, Kim CY, et al. Mitomycin-C induces the apoptosis of human tenon's capsule fibroblast by activation of c-Jun N-terminal kinase 1 and caspase-3 protease. Invest Ophthalmol Vis Sci. 2005; 46:3545–52.
8. Bergstrom TJ, Wilkinson WS, Skuta GL, et al. The effects of subconjunctival mitomycin-C on glaucoma filtration surgery in rabbits. Arch Ophthalmol. 1991; 109:1725–30.

9. Choi JW, Kim TI, Tchah HW. Apoptosis of keratocytes induced by mitomycin C. J Korean Ophthalmol Soc. 2004; 45:490–9.
10. Yu L, Alva A, Su H, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004; 304:1500–2.
11. Gozuacik D, Kimchi A. Autophagy as a cell death and tumor sup-pressor mechanism. Oncogene. 2004; 23:2891–906.

12. Meijer A. Codogno JP. Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol. 2004; 36:2445–62.
Go to : 
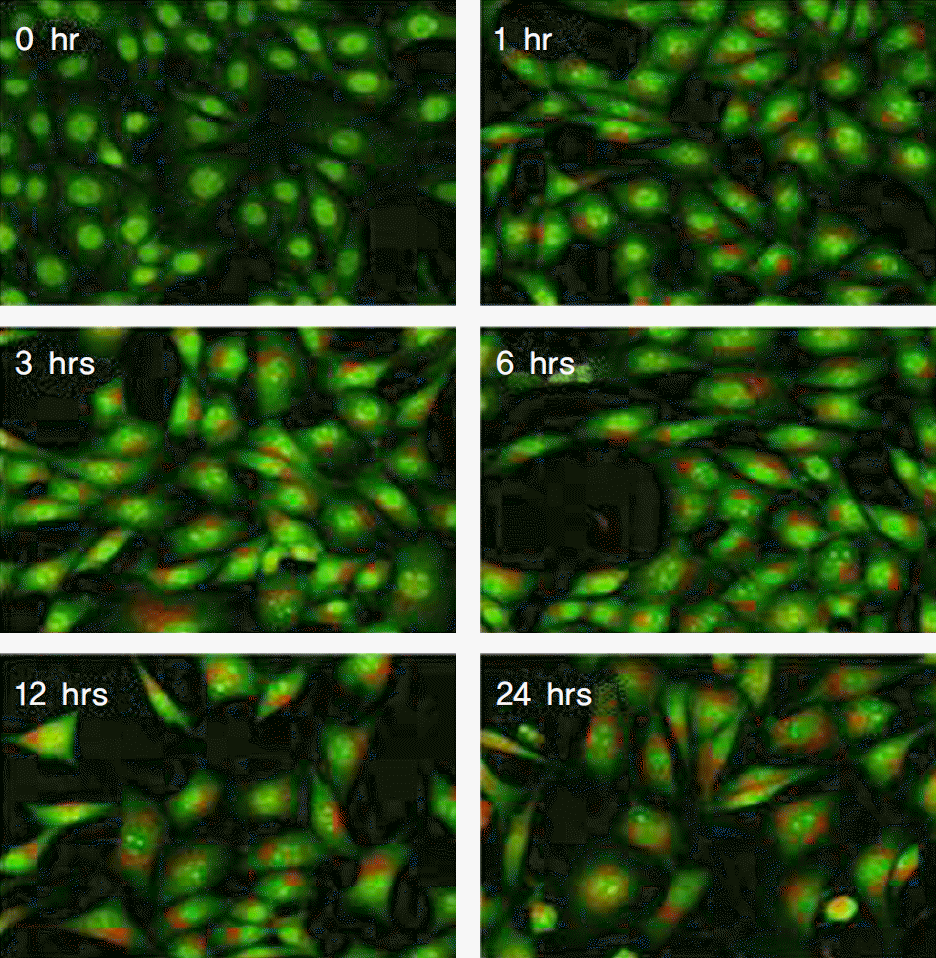 | Figure 1.Mytomycin-C induced an increase of acidic vesicular organelle in human tenon's capsule fibroblasts in a time-dependent manner. Cells were treated with 0.4 mg/ml mitomycin-C for the indicated periods. Cells were stained with acridine orange for 15 min and then the fluorescence intensity of the cells was visualized under fluorescence microscope. |
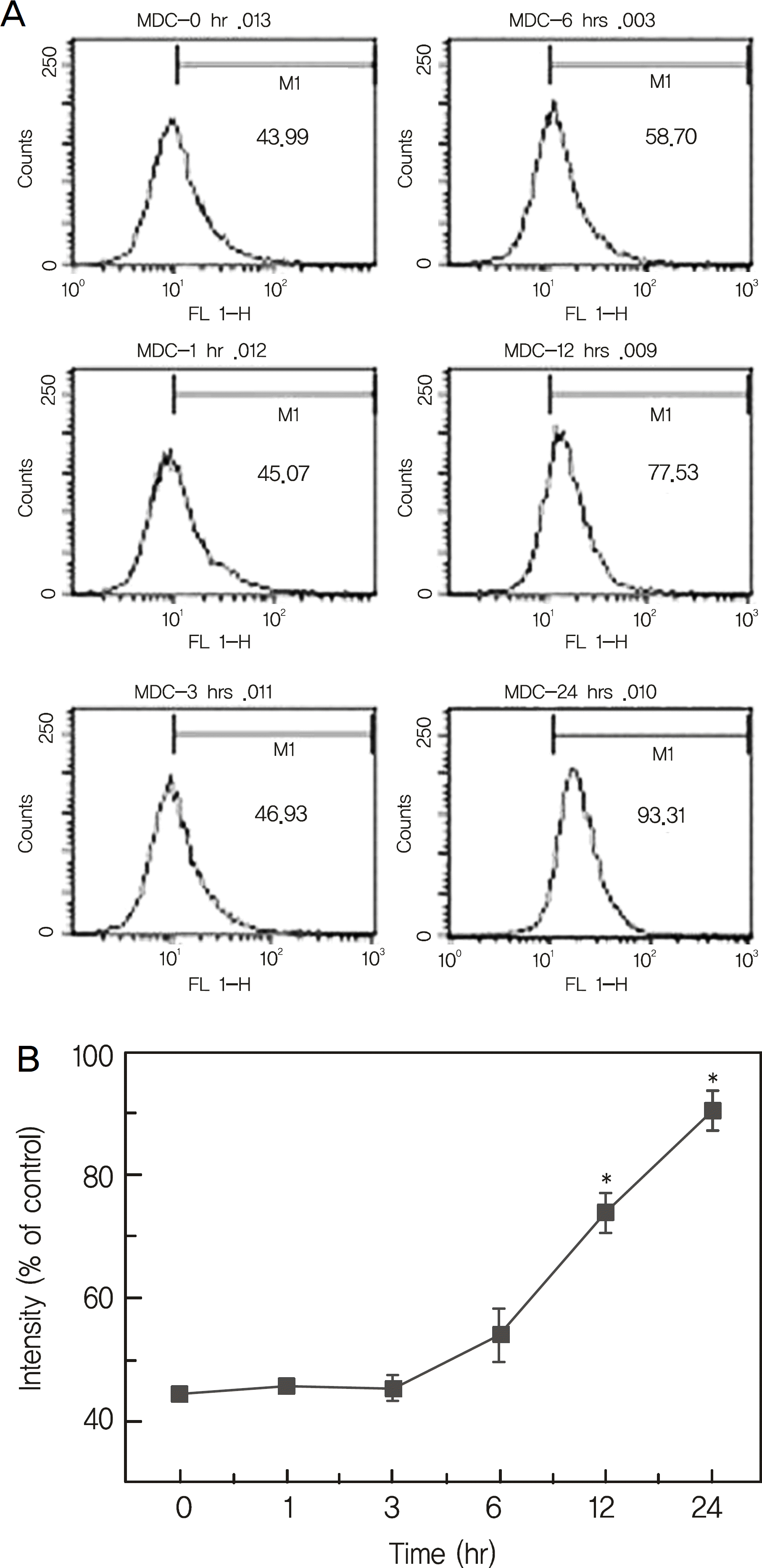 | Figure 2.Mitomycin-C induced an increase of acidic vesicular organelle in human tenon's capsule fibroblasts in a time-de-pendent manner. (A) Cells were treated with 0.4 mg/ml mito-mycin-C for the indicated periods. Cells were stained with monodansylcadaverine for 30 min and then the cells were harvested. After washing out the unbound dye, the cells were subjected to flow cytometric analysis for estimation of intra-cellular acidic vesicular organelle levels. (B) Relative intensities comparing the mitomycin-C treated group with the control group of the FACS analysis. (* p < 0.001 by one-way ANOVA). |
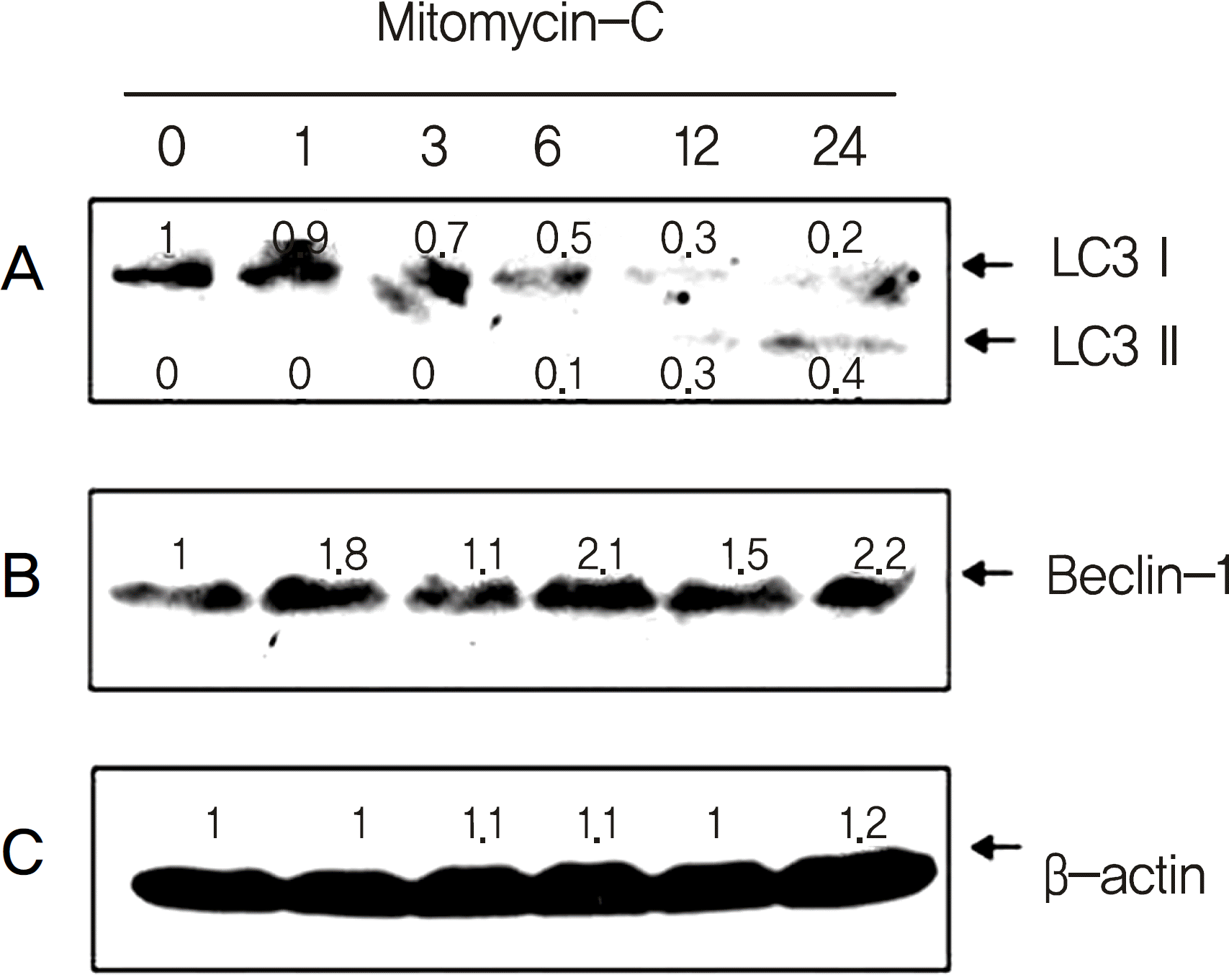 | Figure 3.Mitomycin-C induced both LC3-Ⅱ cleavage and Beclin-1 expression of human tenon's fibroblasts in a time-de-pendent manner. Cells were treated with 0.4 mg/ml mitomy-cin-C for the indicated periods. Cells were separated on 15% SDS-PAGE, transferred onto nitrocellulose membranes, and immunoblotted with anti-LC3 (A), anti-Beclin-1 (B), and anti-β-actin (C) antibodies. The immunoreactive signals were visualized by ECL kit. The protein levels of β-actin were used as the control. |
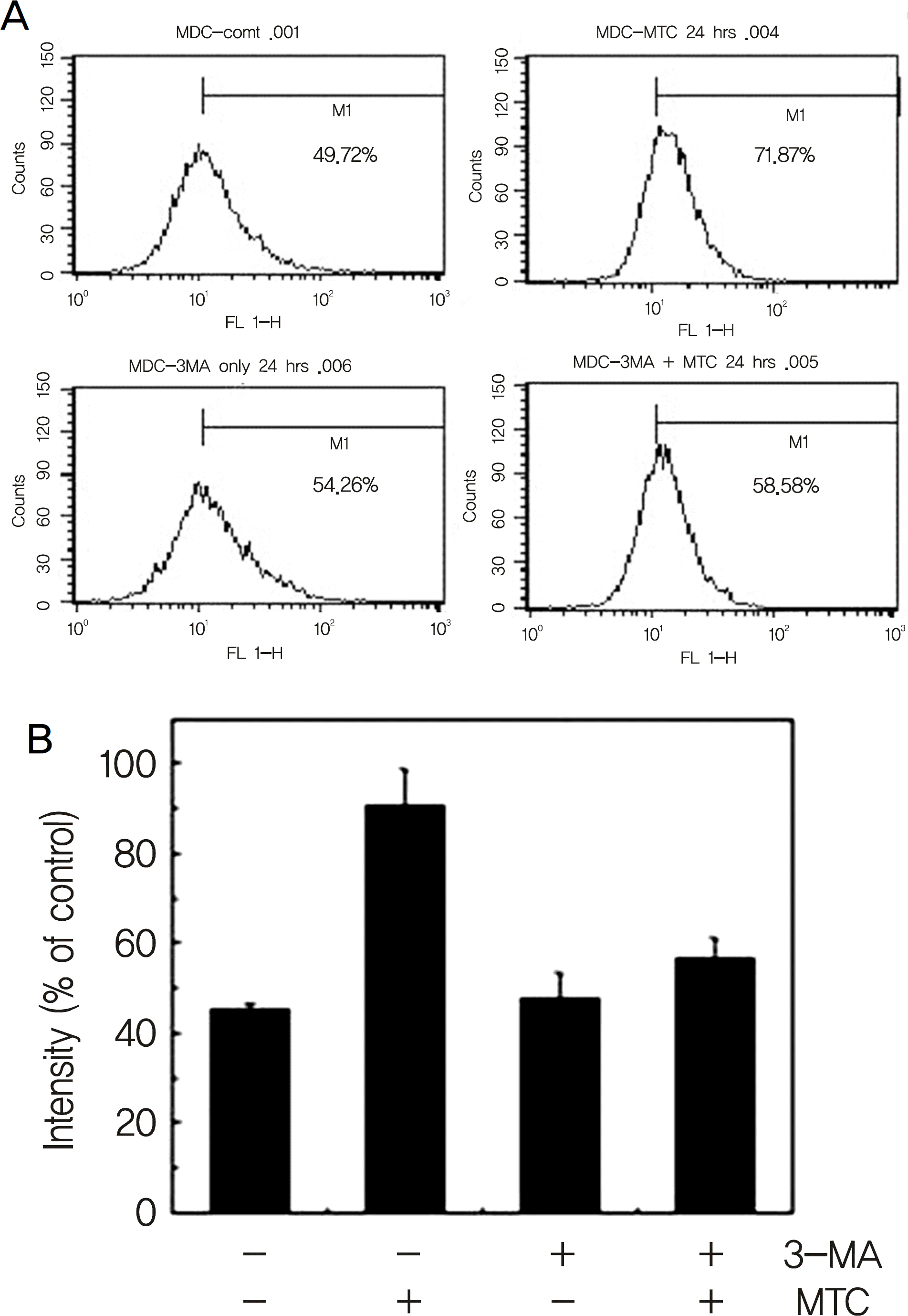 | Figure 4.3-MA inhibited the mitomycin-C induced increase of acidic vesicular organelle in HTCFs. (A) Cells were treated with 0.4 mg/ml mitomycin-C for indicated periods. Cells were stained with monodansylcadaverine for 30 min and then harvested. After washing out the unbound dye, the cells were subjected to flow cytometric analysis for estimation of intra-cellular acidic vesicular organelle levels. (B) Relative intensities of the FACS analysis. (* p < 0.01 by one-way). |
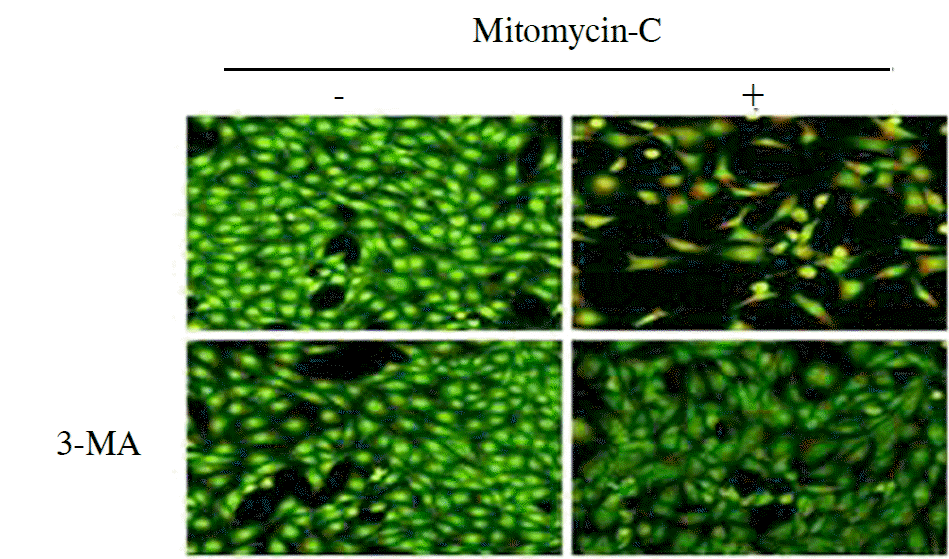 | Figure 5.Fibroblasts were stained with acridine orange for 15 min and then the fluorescence intensity of the cells were visualized under fluorescence microscope. Anti-autophagy agents decreased acidic vesicular organelle. |
 | Figure 6.Apoptosis/autophagy connection in programmed cell death. (A) Autophagy may be indispensable for the occurrence of apoptosis. (B) Autophagy may antagonize apoptosis. (C) Apoptosis and autophagy may occur independently of each other. Inhibition of apoptosis may convert cell death morphology to autophagic and vice versa. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download