Abstract
Purpose
To determine the clinical outcome of intravitreal bevacizumab injection in patients with ischemic central retinal vein obstruction (CRVO).
Methods
The present study was conducted retrospectively on 56 eyes of 56 patients who were diagnosed with CRVO and classified according to ischemic and non-ischemic type and underwent an intravitreal bevacizumab injection. The present study measured changes in visual acuity and central macular thickness, neovascularization in the anterior segment, development of neovascular glaucoma and other clinical complications.
Results
The average number of bevacizumab injections in both groups was 2.07 and 1.62 in the ischemic type. No patients developed neovascular glaucoma in the non-ischemic type group, 14 of 26 eyes in the ischemic type group developed neovascular glaucoma and the mean time to diagnosis was 28.75 weeks. log MAR visual acuity of the ischemic type group prior to injection was 1.56 ± 0.65 on average which improved to 1.44 ± 0.97 (p = 0.45).
Go to : 
References
1. Bashshur ZF, Ma'luf RN, Allam S, et al. Intravitreal triamcinolone for the management of macular edema due to nonischemic central retinal vein occlusion. Arch Ophthalmol. 2004; 122:1137–40.
2. Green WR, Chan CC, Hutchins GM, Terry JM. Central retinal vein occlusion: a prospective histopathologic study of 29 eyes in 28 cases. Trans Am Ophthalmol Soc. 1981; 79:371–422.
3. Pe'er J, Folberg R, Itin A, et al. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology. 1998; 105:412–6.
4. Vinores SA, Youssri Ai, Luna JD, et al. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathol. 1997; 12:99–109.
5. Quinlan PM, Elman MJ, Bhatt AK, et al. The natural course of central retinal vein occlusion. Am J Ophthalmol. 1990; 110:118–23.

6. Magargal LE, Brown GC, Augsburger JJ, Donoso LA. Efficacy of panretinal photocoagulation in preventing neovascular glaucoma following ischemic central retinal vein obstruction. Ophthalmology. 1982; 89:780–4.

7. Guthoff R, Meigen T, Hennemann K, Schrader W. Comparison of bevacizumab and triamcinolone for treatment of macular edema secondary to central retinal vein occlusion a matched pairs analysis. Ophthalmologica. 2010; 224:126–32.
8. Wu WC, Cheng KC, Wu HJ. Intravitreal triamcinolone acetonide vs bevacizumab for treatment of macular oedema due to central retinal vein occlusion. Eye. 2009; 23:2215–22.

9. Hayreh SS, Rojas P, Podhajsky P, et al. Ocular neovascularization with retinal vascular occlusion III. Incidence of ocular neovascularization with retinal vein occulusion. Ophthalmology. 1983; 90:488–506.
10. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. The Central Vein Occlusion study Group M report. Ophthalmology. 1995; 102:1425–33.
11. McAllister IL, Douglas JP, Constable IJ, Yu DY. Laser-induced chorioretinal venous anastomosis for nonischemic central retinal vein occlusion: evaluation of the complications and their risk factors. Am J Ophthalmol. 1998; 126:219–29.

12. Elman MJ, Raden RZ, Carrigan A. Intravitreal injection of tissue plasminogen activator for central retinal vein occlusion. Trans Am Ophthalmol Soc. 2001; 99:219–21.

13. Opremcak EM, Bruce RA, Lomeo M, et al. Radial optic neurotomy for central retinal vein occlusion: a retrospective pilot study of 11 consecutive cases. Retina. 2001; 21:408–15.
14. Weiss JN, Bynoe LA. Injection of tissue plasminogen activator into a branch retinal vein in eyes with central retinal vein occlusion. Ophthalmology. 2001; 108:2249–57.

15. Jonas JB, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide as treatment of macular edema in central retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2002; 240:782–3.

16. Greenberg PB, Martidis A, Rogers AH, et al. Intravitreal triamcinolone acetonide for macular oedema due to central retinal vein occlusion. Br J Ophthalmol. 2002; 86:247–8.

17. Ip MS, Kumar KS. Intravitreous triamcinolone acetonide as treatment for macular edema from central retinal vein occlusion. Arch Ophthalmol. 2002; 120:1217–9.

18. Ip MS, Gottlieb JL, Kahana A, et al. Intravitreal triamcinolone for the treatment of macular edema associated with central retinal vein occlusion. Arch Ophthalmol. 2004; 122:1131–6.
19. Iturralde D, Spaide RF, Meyerle CB, et al. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006; 26:279–84.
20. Schaal KB, Höh AE, Scheuerle A, et al. Bevacizumab for the treatment of macular edema secondary to retinal vein occlusion. Ophthalmology. 2007; 104:285–9.
21. Choi SW, Kim HW, Yun IH. Intravitreal bevacizumab treatment of macular edema in central retinal vein occlusion. J Korean Ophthalmol Soc. 2010; 51:707–15.

22. Shin SG, Ahn JH, Rho SH. A clinical analysis on 456 cases of glaucoma among outpatients during 5 years. J Korean Ophthalmol Soc. 1987; 28:1021–6.
23. Kim DG, Kim HJ, Song MS. Clinical study on glaucomatous patients. J Korean Ophthalmol Soc. 1989; 30:755–9.
24. Song GW, Jin KH, Kim JM. Clinical data on glaucomatous patients. J Korean Ophthalmol Soc. 1990; 31:1179–83.
25. Hwang IC, Jeong SK, Yang KJ. A clinical study on glaucoma. J Korean Ophthalmol Soc. 1992; 33:394–400.
26. Lee CK, Cho YJ, Hong YJ. Distribution of glaucoma outpatients. J Korean Ophthalmol Soc. 1995; 36:1020–7.
27. Lee CH, Jin GH, Kim DM. Clinical analysis on glaucoma. J Korean Ophthalmol Soc. 1998; 39:362–8.
Go to : 
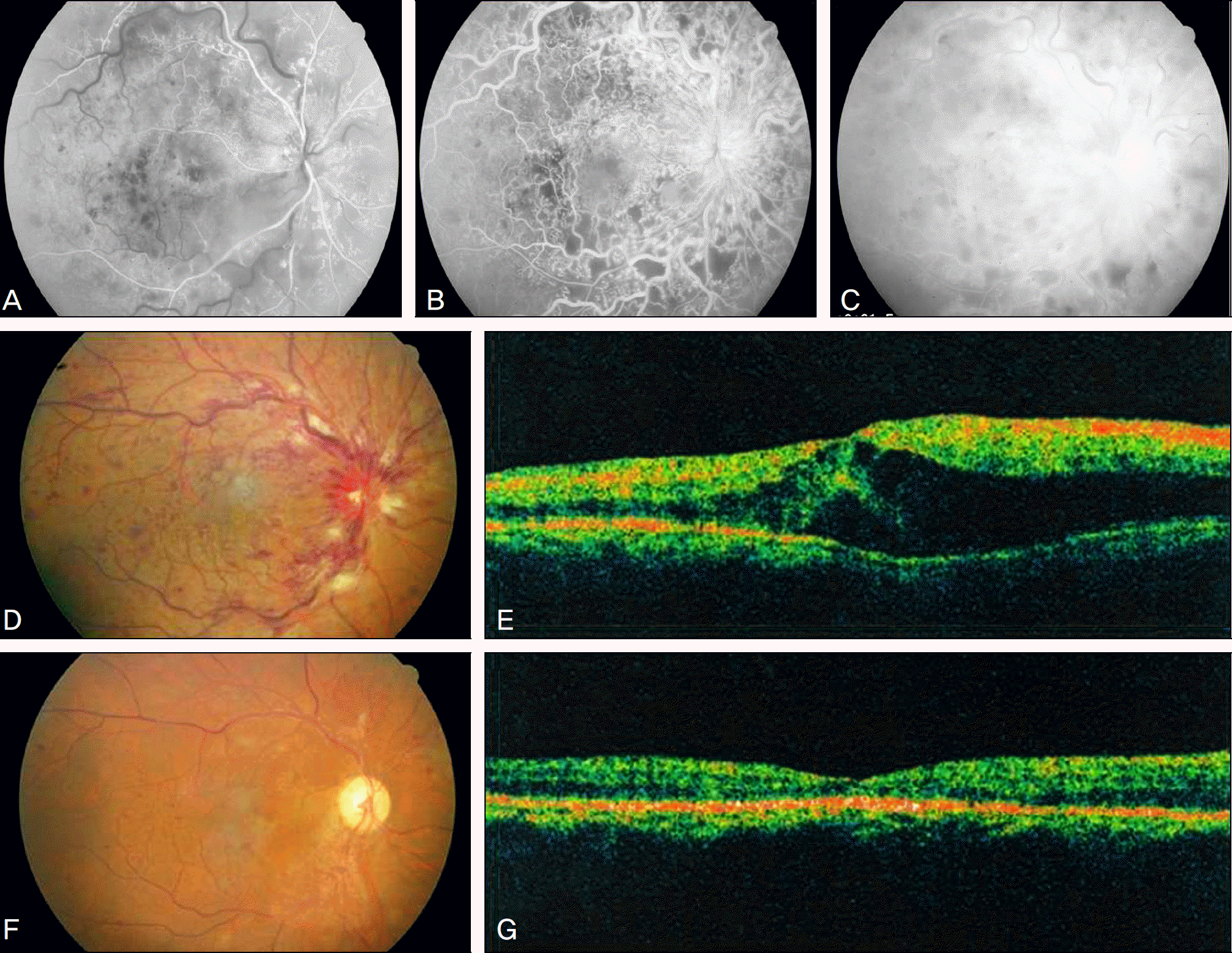 | Figure 1.A 52 year old man visited our clinic for visual disturbance. Best corrected visual acuity was 0.9 log MAR scale. FAG in the arterial and venous phase showed extensive hypoperfusion and blocked fluorescence at the post pole (A, B), and hypoperfusion with diffuse leakage in the late phase (C). Fundus photograph (D) showed multiple retinal hemorrhage and cotton wool spots along the major vascular arcade, OCT (E) showed diffuse cystoid macular edema before intravitreal bevacizumab injection (CMT: 522 μ m). 6 months after the injection, fundus photograph (F) showed that retinal hemorrhage and cotton wool spots had decreased, OCT (G) showed decreased macular edema after intravitreal bevacizumab injection (CMT: 174 μ m). |
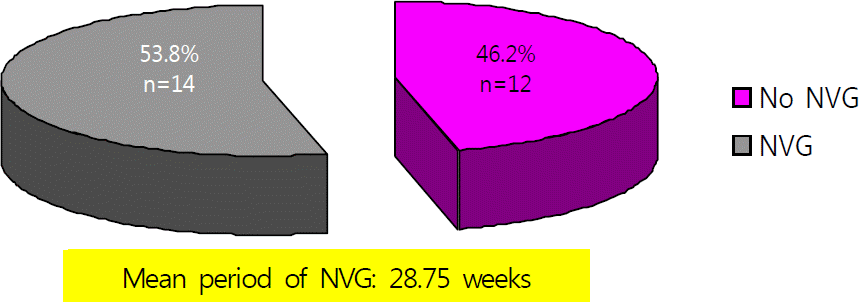 | Figure 2.This diagram shows the occurrence of neovascular glaucoma (NVG) in patients of ischemic central retinal vein obstruction, mean duration of neovascular glaucoma was 28.75 weeks, Ahmed valve implantation and panretinal photocoagulation was performed on 1 patient and 8 patients respectively. |
Table 1.
Baseline dermographics of total patients
| factor | Total patients (n = 56) | Non-ischemic CRVO‡ (n = 30) | (n = 26) Ischemic CRVO‡ |
|---|---|---|---|
| Sex (M:F) | 37:19 | 20:10 | 17:9 |
| Age (mean ± SD, yr) | 55.23 ± 11.38 | 50.67 ± 9.80 | 60.5 ± 10.93 |
| Hypertension (yes:no) | 40:16 | 26:4 | 14:12 |
| Diabetes mellitus (yes:no) | 7:49 | 2:28 | 5:21 |
| BCVA* (mean ± SD, log MAR) | 1.05 ± 0.70 | 0.64 ± 0.39 | 1.56 ± 0.65 |
| CMT† (mean ± SD, μ m) | 611.84 ± 259.33 | 590.50 ± 231.37 | 699.79 ± 305.67 |
| Mean follow up periods (mon) | 15.20 | 15.53 | 17.84 |
| Mean times of bevacizumab injection | 2.07 | 2.47 | 1.62 |
Table 2.
Changes in best corrected visual acuity (log MAR)
| |
Non-ischemic CRVO* (n = 30) |
Ischemic CRVO* (n = 26) |
||
|---|---|---|---|---|
| BCVA† (mean ± SD) | p-value‡ | BCVA† (mean ± SD) | p-value‡ | |
| Prior injection | 0.64 ± 0.39 | | 1.56 ± 0.65 | |
| 1 month after injection | 0.59 ± 0.49 | 0.11 | 1.52 ± 0.22 | 0.53 |
| 6 months after injection | 0.54 ± 0.55 | 0.12 | 1.49 ± 0.35 | 0.46 |
| 12 months after injection | 0.45 ± 0.55 | 0.05 | 1.44 ± 0.93 | 0.45 |
Table 3.
Changes in central macular thickness
| |
Non-ischemic CRVO* (n = 30) |
Ischemic CRVO* (n = 26) |
||
|---|---|---|---|---|
| CMT† (mean ± SD) | p-value‡ | CMT† (mean ± SD) | p-value† | |
| Prior injection | 590.50 ± 231.37 | | 699.79 ± 305.67 | |
| 1 month after injection | 475.10 ± 290.88 | 0.31 | 525.07 ± 374.66 | 0.19 |
| 6 months after injection | 418.83 ± 147.22 | 0.44 | 479.88 ± 334.69 | 0.12 |
| 12 months after injection | 375.12 ± 231.37 | 0.33 | 411.00 ± 338.83 | 0.01 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download