Abstract
Purpose
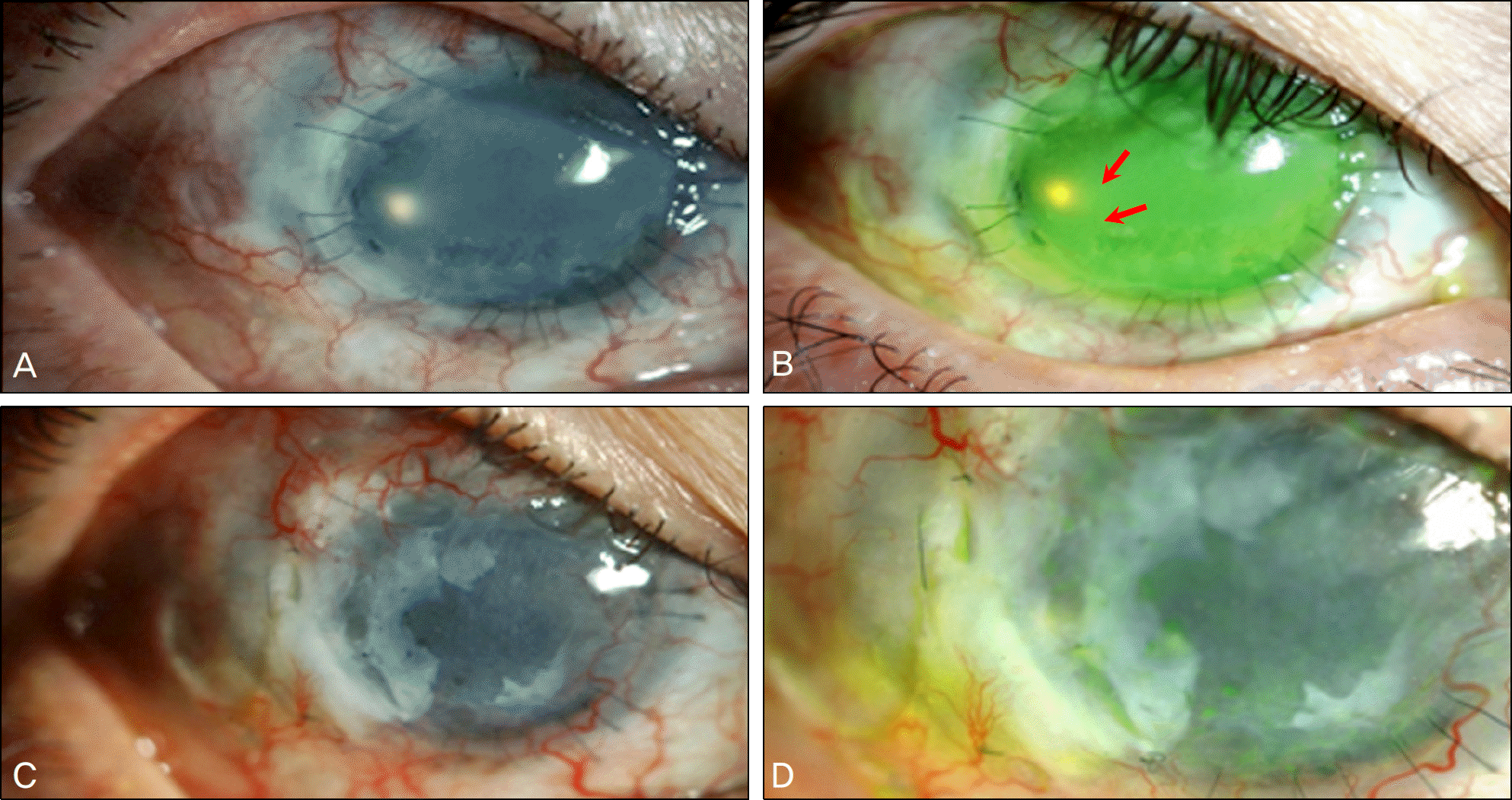
To report the use of amniotic membrane (AM) as a mesenchymal stem cell (MSC) carrier in cornea perforation with severe limbal stem cell deficiency due to a chemical burn on both eyes.
Case summary
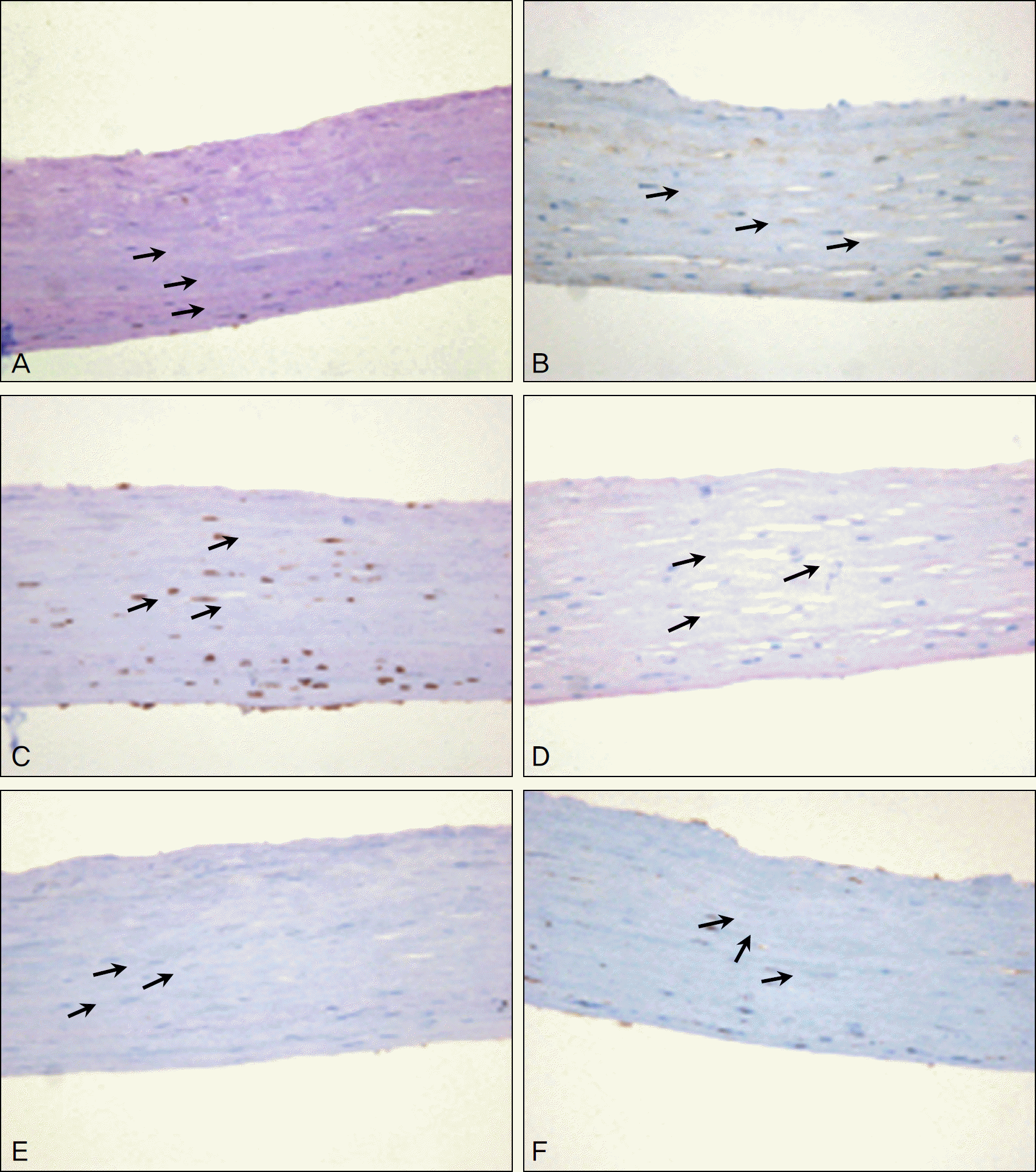
The patient in the present study is a 43-year-old man with a history of bilateral alkaline chemical injury six years previously. The patient suffered from total limbal stem cell deficiency (LSCD) in the injured eyes and underwent numerous operations including autologous conjunctival flap, AM graft, penetrating keratoplasty, keratolimbal autograft and ex vivo expanded limbal allogrft. However, the patient's ocular condition deteriorated due to persistent LSCD, which led to corneal perforation resulting in the need for an urgent therapeutic operation. In the patient's past ocular history, Ahmed valve implantation was performed for uncontrolled IOP. Revision of the Ahmed tube due to exposure and conjunctival erosion was performed again via placement of an AM over the exposed tube one year previous to presentation to our hospital. The authors performed a transplanted AM graft at the cornea perforation using sub-conjunctival AM believed to contain MSCs. Six months postoperatively, the ocular surface was well mainatained and the graft remained healthy. Transplanted AM showed morphological and immunohistochemical findings similar to those of MSCs.
References
1. Zieske JD, Bukusoglu G, Gipson IK. Enhancement of vinculin synthesis by migrating stratified squamous epithelium. J Cell Biology. 1989; 109:571–6.

2. Chung EH, Hutcheon AE, Joyce NC, Zieske JD. Synchronization of the G1/S transition in response to corneal debridement. Invest Ophthalmol Vis Sci. 1999; 40:1952–8.
3. Hall PA, Watt FM. Stem cells: the generation and maintenance of cellular diversity. Development. 1989; 106:619–33.

4. Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983; 24:1442–3.
5. Sandvig KU, Haaskjold E, Bjerknes R, et al. Cell kinetics of conjunctival and corneal epithelium during regeneration of different-sized corneal epithelial defects. Acta Ophthalmol. 1994; 172:43–8.

6. Kotton DN, Ma BY, Cardoso WV, et al. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001; 128:5181–8.

7. Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998; 279:1528–30.

8. Okamoto R, Yajima T, Yamazaki M, et al. Damaged epithelia regenerated by bone marrow-derived cells in the human gastro-intestinal tract. Nat Med. 2002; 8:1011–7.

9. Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989; 96:709–23.

10. Tsai RJ, Tseng SC. Human allograft limbal transplantation for corneal surface reconstruction. Cornea. 1994; 13:389–400.

11. Kwitko S, Marinho D, Barcaro S, et al. Allograft conjunctival transplantation for bilateral ocular surface disorders. Ophthalmology. 1995; 102:1020–5.

12. Shimazaki J, Aiba M, Goto E, et al. Transplantation of human limbal epithelium cultivated on amniotic membrane for the treatment of severe ocular surface disorders. Ophthalmology. 2002; 109:1285–90.
13. Ti SE, Grueterich M, Espana EM, et al. Correlation of long term phenotypic and clinical outcomes following limbal epithelial transplantation cultivated on amniotic membrane in rabbits. Br J Ophthalmol. 2004; 88:422–7.

14. Park GS, Je J, Kim JC. Stepwise surgical approach for in vivo expansion of epithelial stem cells to treating severe acute chemical burns with total limbal deficiency. Korean J Ophthalmol. 2003; 17:75–82.
15. Jenkins C, Tuft S, Liu C, Buckley R. Limbal transplantation in the management of chronic contact lens-associated epitheliopathy. Eye. 1993; 7:629–33.
16. Coster DJ, Aggarwal RK, Williams KA. Surgical management of ocular surface disorders using conjunctival and stem cell allografts. Br J Ophthalmol. 1995; 79:977–82.

17. Holland EJ, Schwartz GS. The evolution of epithelial transplantation for severe ocular surface disease and a proposed classification system. Cornea. 1996; 15:549–56.

18. Henderson TR, Coster DJ, Williams KA. The long term outcome of limbal allografts: the search for surviving cells. Br J Ophthalmol. 2001; 85:604–9.

19. Pels E, van der Gaag R. HLA-A,B,C, and HLA-DR antigens and dendritic cells in fresh and organ culture preserved corneas. Cornea. 1984; 3:231–9.

20. Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated human epithelium. Lancet. 1997; 349:990–3.
21. Koizumi N, Inatomi T, Quantock AJ, et al. Amniotic membrane as a substrate for cultivating limbal corneal epithelial cells for autologous transplantation in rabbits. Cornea. 2000; 19:65–71.

22. Rama P, Bonini S, Lambiase A, et al. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation. 2001; 72:1478–85.
23. Prabhasawat P, Tesavibul N, Komolsuradej W. Single and multilayer amniotic membrane transplantation for persistent corneal epithelial defect with and without stromal thinning and perforation. Br J Ophthalmol. 2001; 85:1455–63.

24. Shin KS, Chung IY, Seo SW. The effect of amniotic membrane transplantation for corneal ulcer and ocular surface diseases. J Korean Ophthalmol Soc. 2003; 44:1305–10.
25. Tseng SC, Prabhasawat P, Lee SH. Amniotic membrane transplantation for conjunctival surface reconstruction. Am J Ophthalmol. 1997; 124:765–74.

26. Tamhane A, Vajpayee RB, Biswas NR, et al. Evaluation of amniotic membrane transplantation as an adjunct to medical therapy as compared with medical therapy alone in acute ocular burns. Ophthalmology. 2005; 112:1963–9.

27. Meller D, Pires RT, Mack RJ, et al. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000; 107:980–9.

28. Tseng SC, Prabhasawat P, Barton K, et al. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998; 116:431–41.

29. Ucakhan OO, Köklü G, Firat E. Nonpreserved human amniotic membrane transplantation in acute and chronic chemical eye injuries. Cornea. 2002; 21:169–72.

30. Hanada K, Shimazaki J, Shimmura S, Tsubota K. Multilayered amniotic membrane transplantation for severe ulceration of the cornea and sclera. Am J Ophthalmol. 2001; 131:324–31.

31. Kicic A, Shen W, Rakoczy PE. The potential of marrow stromal cells in stem cell therapy. Eye. 2001; 15:695–707.

32. Shin MS, Hong HS, Son YS, Kim JC. Effects of damaged human corneal epithelial cells on differentiation of human mesenchymal stem Cell. J Korean Ophthalmol Soc. 2007; 48:423–30.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download