Abstract
Purpose
To investigate the clinical manifestation and response to high-dose steroid therapy in Korean optic neuritis patients.
Methods
We retrospectively reviewed the medical records of 13 patients diagnosed with optic neuritis who were treated with high-dose steroid and who were followed-up for more than three months in Seoul National University Hospital between January 2005 and December 2008.
Results
Pain on extraocular movement (EOM) and disc swelling were observed in 61% (8/13) patients diagnosed with optic neuritis. Visual acuity (VA) improved to more than 20/40 in 77% (10/13) of patients after high-dose steroid therapy. The patients who resolved within one month recovered VA a mean of 18 days after onset. The recovery period had nothing to do with the initial time of high-dose steroid therapy.
Go to : 
References
1. Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol. 2008; 65:727–32.
2. Beck RW, Gal RL, Bhatti MT, et al. Visual function more than 10 years after optic neuritis: experience of the optic neuritis treatment trial. Am J Ophthalmol. 2004; 137:77–83.
3. Visual function 5 years after optic neuritis: experience of the Optic Neuritis Treatment Trial. The Optic Neuritis Study Group. Arch Ophthalmol. 1997; 115:1545–52.
4. Rizzo JF 3rd, Lessell S. Risk of developing multiple sclerosis after uncomplicated optic neuritis: a long-term prospective study. Neurology. 1988; 38:185–90.
5. Beck RW, Cleary PA. Optic neuritis treatment trial. One-year follow-up results. Arch Ophthalmol. 1993; 111:773–5.
6. Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000; 343:1430–8.

8. Isayama Y, Takahashi T, Shimoyoma T, Yamadori A. Acute optic neuritis and multiple sclerosis. Neurology. 1982; 32:73–6.

9. Lin YC, Yen MY, Hsu WM, et al. Low conversion rate to multiple sclerosis in idiopathic optic neuritis patients in Taiwan. Jpn J Ophthalmol. 2006; 50:170–5.

10. Wang JC, Tow S, Aung T, et al. The presentation, aetiology, management and outcome of optic neuritis in an Asian population. Clin Experiment Ophthalmol. 2001; 29:312–5.

11. Lim SA, Goh KY, Tow S, et al. Optic neuritis in Singapore. Singapore Med J. 2008; 49:667–71.
12. Wakakura M, Minei-Higa R, Oono S, et al. Baseline features of idiopathic optic neuritis as determined by a multicenter treatment trial in Japan. Optic Neuritis Treatment Trial Multicenter Cooperative Research Group (ONMRG). Jpn J Ophthalmol. 1999; 43:127–32.
13. Lee YJ, Chang BL. Clinical manifestation of optic neuritis. J Korean Ophthalmol Soc. 1997; 38:1969–74.
14. Ahn BC, Kim HS, Ahn HS. Clinical profile of the optic neuritis in Korea. J Korean Ophthalmol Soc. 1997; 38:1827–33.
15. Beck RW, Cleary PA, Anderson MM Jr, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med. 1992; 326:581–8.
16. Beck RW, Trobe JD, Moke PS, et al. High- and low-risk profiles for the development of multiple sclerosis within 10 years after optic neuritis: experience of the optic neuritis treatment trial. Arch Ophthalmol. 2003; 121:944–9.
17. Wakakura M, Mashimo K, Oono S, et al. Multicenter clinical trial for evaluating methylprednisolone pulse treatment of idiopathic optic neuritis in Japan. Optic Neuritis Treatment Trial Multicenter Cooperative Research Group (ONMRG). Jpn J Ophthalmol. 1999; 43:133–8.
18. The clinical profile of optic neuritis. Experience of the Optic Neuritis Treatment Trial. Optic Neuritis Study Group. Arch Ophthalmol. 1991; 109:1673–8.
19. Chan CK, Lam DS. Optic neuritis treatment trial:10-year follow-up results. Am J Ophthalmol. 2004; 138:695.

Go to : 
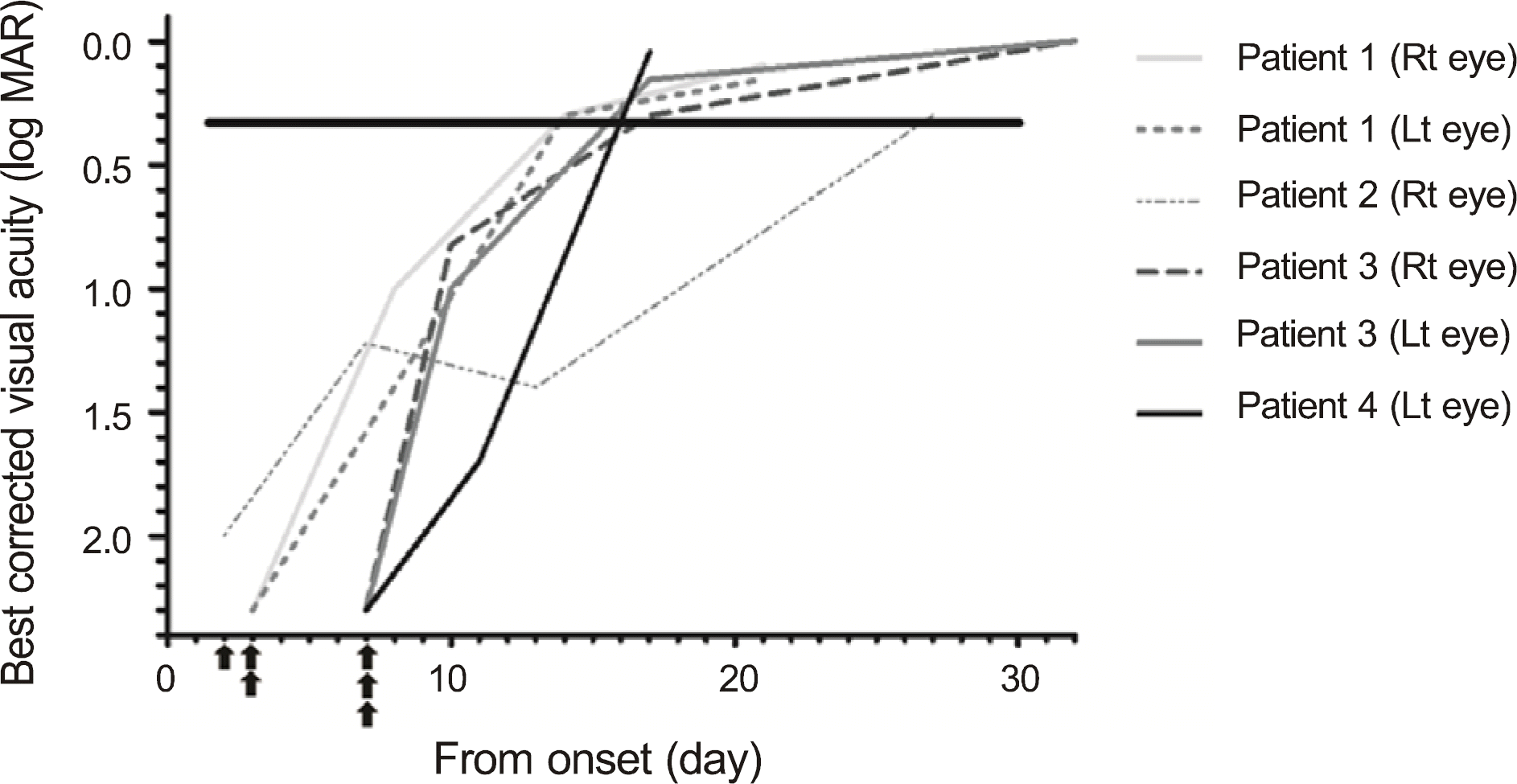 | Figure 1.Improvement in visual acuity in early pulse steroid treatment group. ▲ Time to start steroid pulse therapy. |
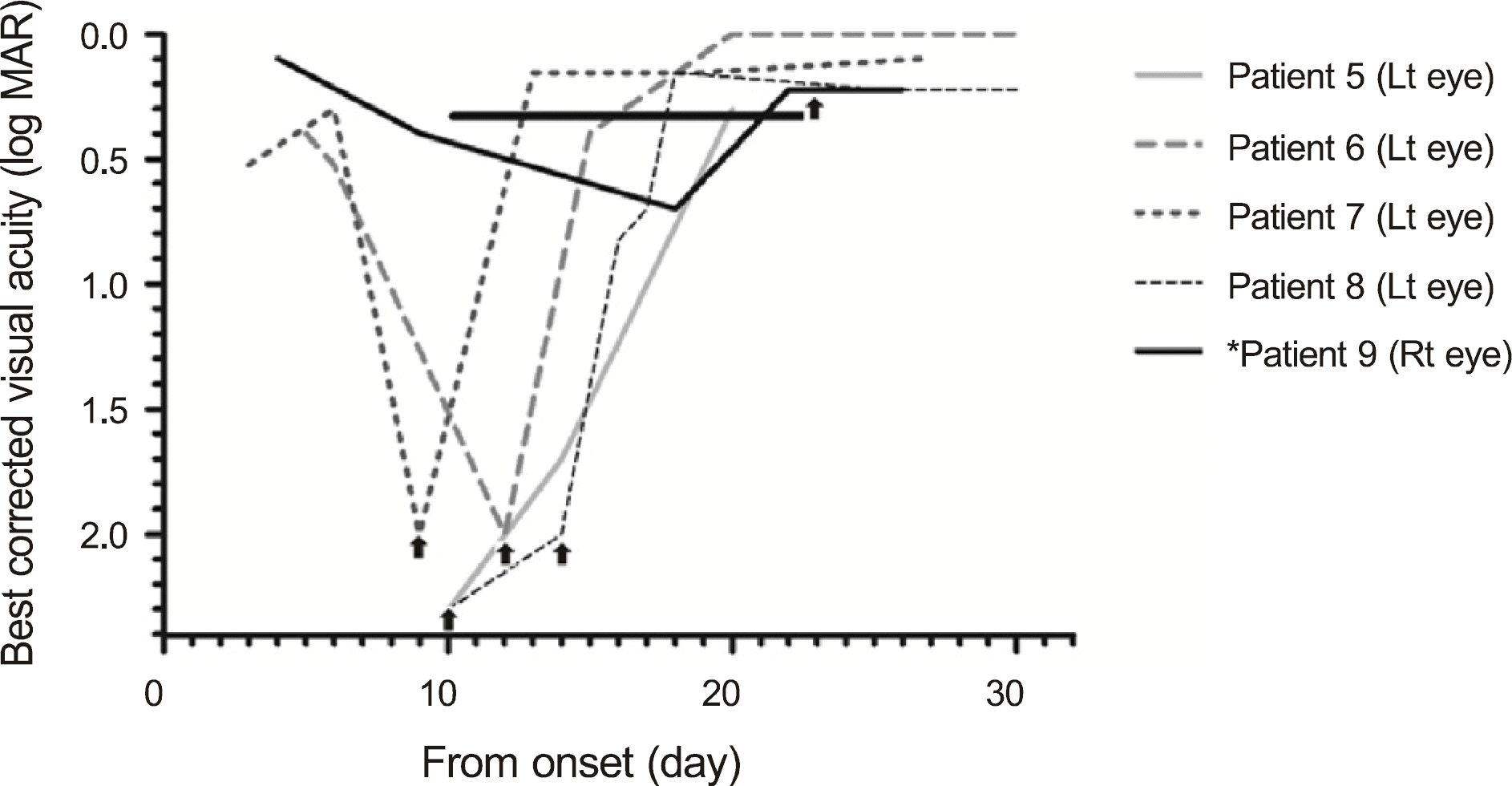 | Figure 2.Improvement in visual acuity in late pulse steroid treatment group. ▲ Time to start steroid pulse therapy.* Patient 9 received pulse steroid treatment, although her visual acuity of the right eye was 0.6, because the visual acuity of her left eye was not improved for 23 days without any treatment. |
Table 1.
Demographics of patients
| Characteristics | Number of patients (%) |
|---|---|
| Sex | |
| Male | 4 |
| Female | 9 |
| Age (yr) | |
| Mean ± SD | 39.6 ± 11.1 |
| Range | 16–55 |
| Laterality | |
| Mono-ocular | 9 |
| Binocular | 4 |
| Recurrence | |
| Primary cases | 9 |
| Recurrent cases | 4 |
Table 2.
Clinical characteristics of patients
| Characteristics | Number of patients or eyes (%) |
|---|---|
| The worst visual acuity (eye) | |
| 0.003 | 5 (29) |
| 0.005 ≤ < 0.01 | 7 (41) |
| 0.01 ≤ < 0.02 | 3 (17) |
| 0.02 ≤ < 0.1 | 0 (0) |
| 0.1 ≤ | 2 (12) |
| Disc swelling (patient) | 8 (61) |
| * EOM pain (patient) | 8 (61) |
| † Abnormal MRI finding (patient) | 10 (77) |
| History of ‡ URI (patient) | 6 (46) |
Table 3.
Comparison between early and late pulse steroid treatment groups
| | * Early treatment group (Group A) | † Late treatment group (Group B) | ‡ p-value |
|---|---|---|---|
| Number of patients & eyes | 4 patients 6 eyes | 5 patients 5 eyes | |
| Time from onset to pulse steroid treatment (mean ± SD, day) | 4.83 ± 2.40§ (5, 2–7) | 14.2 ± 5.21 (14, 9–22) | 0.006 |
| Time from steroid treatment to recovery (mean ± SD, day) | 12.83 ± 5.98 (11, 10–25) | 4.4 ± 3.58 (4, 0–10) | 0.011 |
| Time from onset to recovery (mean ± SD, day) | 17.66 ± 4.80 (17, 14–27) | 18.6 ± 3.43 (20, 13–22) | 0.355 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download