Abstract
Methods
Literature review using the Korean medical database and the Korean Ophthalmological Society database was performed. Studies used consisted of patients with diabetic macular edema, comparing intravitreal triamcinolone acetonide (IVTA) injection with posterior subtenon triamcinolone acetonide (STTA) injection or intravitreal bevacizumab (IVB) injection, according to visual acuity (VA) outcomes, central macular thickness (CMT), and intraocular pressure (IOP) at 1, 3, and 6 months.
Results
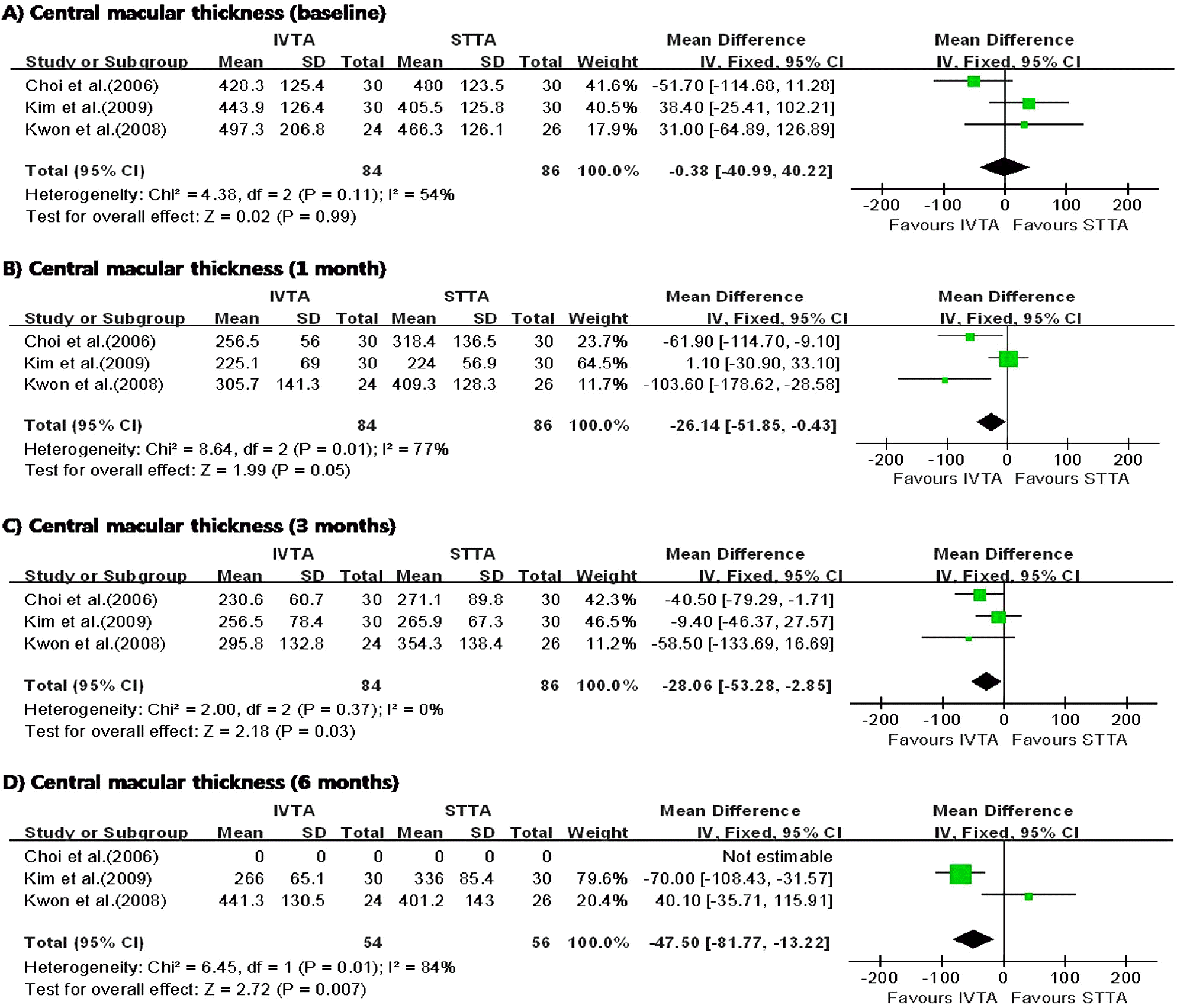
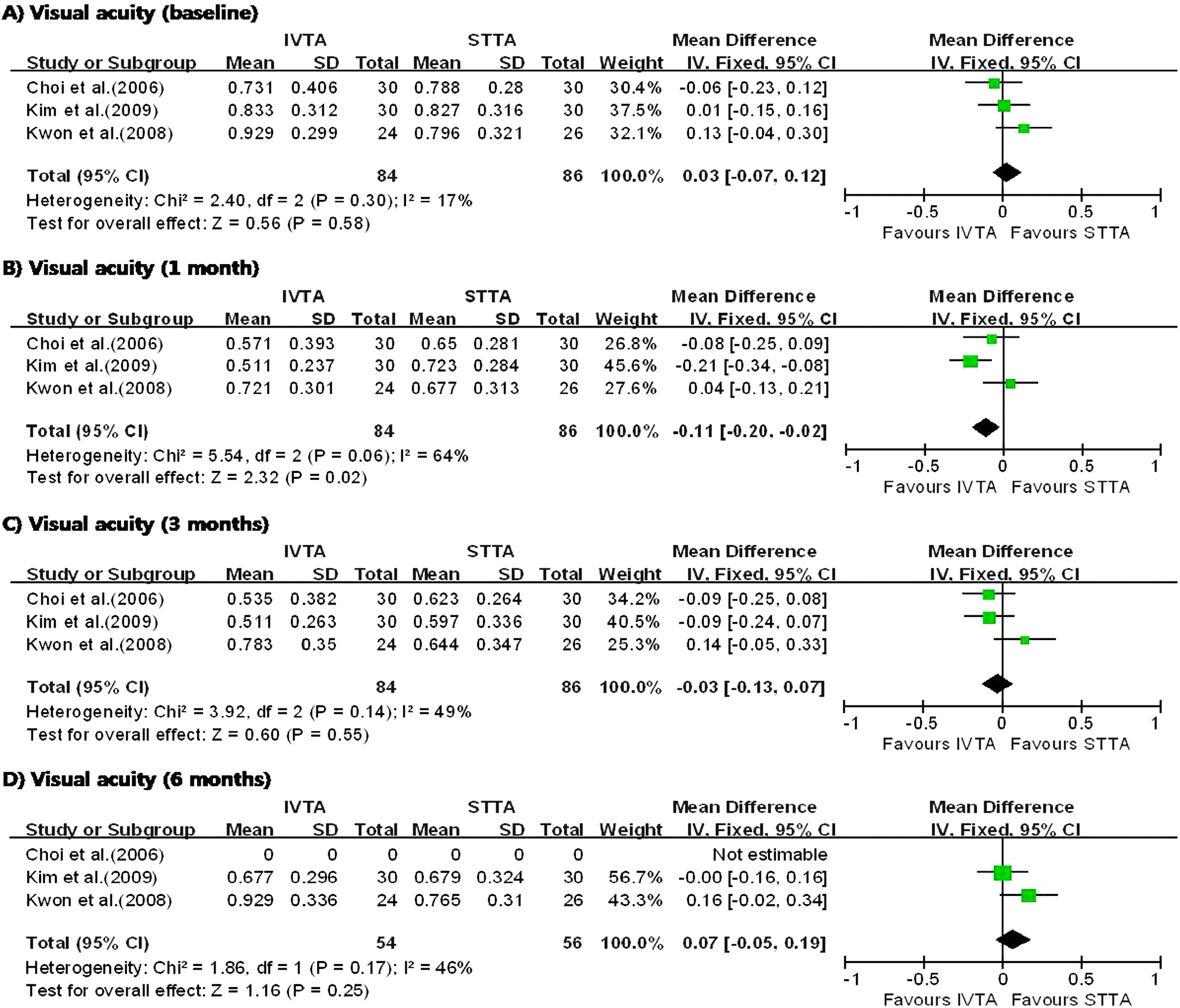
I n the three studies comparing IVTA injection with STTA injection, IVTA injection demonstrated greater improvement in VA at 1 month and CMT at 6 months. The patients who received IVTA injection had significantly higher IOP at 3 months. In the three studies comparing IVTA injection with IVB injection, IVTA injection demonstrated greater improvement in VA at 3 months and CMT at 6 months.
Go to : 
References
1. Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. ETDRS report number 1. Arch Ophthalmol. 1985; 103:1796–806.
2. Early Treatment Diabetic Retinopathy Study Research Group. Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology. 1991; 98:741–56.
3. Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The longterm incidence of macular edema. Ophthalmology. 1995; 102:7–16.
4. Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photo-coagulation for diabetic macular edema. Ophthalmology. 2008; 115:1447–9.
5. Early Treatment Diabetic Retinopathy Study Research Group. Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report number 19. Ophthalmology. 1995; 113:1144–55.
6. Bresnick GH. Diabetic maculopathy: a critical review highlighting diffuse macular edema. Ophthalmology. 1983; 90:1301–17.
7. Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002; 109:920–7.

8. Sutter FK, Simpson JM, Gillies MC. Intravitreal triamcinolone for diabetic macular edema that persists after laser treatment: three-month efficacy and safety results of a prospective, randomized, double-masked, placebocontrolled clinical trial. Ophthalmology. 2004; 111:2044–9.
9. Bonini-Filho MA, Jorge R, Barbosa JC, et al. Intravitreal injection versus sub-Tenon's infusion of triamcinolone acetonide for refractory diabetic macular edema: a randomized clinical trial. Invest Ophthalmol Vis Sci. 2005; 46:3845–9.

10. Cunningham ET Jr, Adamis AP, Altaweel M, et al. Macugen Diabetic Retinopathy Study Group. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005; 112:1747–57.
11. Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, et al. Pan-American Collaborative Retina Study Group. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007; 114:743–50.
12. Chun DW, Heier JS, Topping TM, et al. A pilot study of multiple intravitreal injections of ranibizumab in patients with center-involving clinically significant diabetic macular edema. Ophthalmology. 2006; 113:1706–12.

13. Kim HD, Choi KS, Lee SJ. Combined therapy of intravitreal bevacizumab and posterior subtenon triamcinolone acetonide injection in diabetic macular edema. J Korean Ophthalmol Soc. 2009; 50:1652–6.

14. Choi YJ, Oh IK, Oh JR, Huh K. Intravitreal versus posterior subtenon injection of triamcinolone acetonide for diabetic macular edema. Korean J Ophthalmol. 2006; 20:205–9.

15. Kwon SJ, Shin JP, Kim SY. Intravitreal versus subtenon injection of triamcinolone acetonide for diabetic macular edema. J Korean Ophthalmol Soc. 2008; 49:81–90.
16. Lee JW, Lee BH, Lim JH, Lee KW. Intravitreal triamcinolone versus bevacizumab for treatment of diabetic macular edema. J Korean Ophthalmol Soc. 2009; 50:1184–9.

17. Oh SB, Moon JW, Kim HC. Comparison of effects of intravitreal triamcinolone and bevacizumab in the treatment of diabetic macular edema. J Korean Ophthalmol Soc. 2009; 50:1190–6.

18. Hwang YH, Moon SW. Intravitreal and additional posterior subtenon triamcinolone injection in diabetic macular edema. J Korean Ophthalmol Soc. 2007; 48:506–12.
19. Chang MW, Kim SW, Oh IK, et al. Intravitreal triamcinolone injection with or without bevacizumab for diabetic macular edema. J Korean Ophthalmol Soc. 2008; 49:1269–74.

20. Oh MJ, Choi KR, Lee SY, Lee JH. Clinical manifestations of intraocular pressure elevation after intravitreal injection of triamcinolone acetonide. J Korean Ophthalmol Soc. 2006; 47:1575–82.
21. Jung JW, Nam DH, Shyn KH. The complications after intravitreal injection of triamcinolone acetonide. J Korean Ophthalmol Soc. 2007; 48:55–62.
22. Kim TW, Park SP, Yu HG. A case of intralenticular triamcinolone complicated by intravitreal triamcinolone acetonide injection. J Korean Ophthalmol Soc. 2005; 46:1747–50.
23. Jeong BJ, Seo HD, Kim KH, et al. Triamcinolone regurgitation into anterior chamber after intravitreal triamcinolone injection. J Korean Ophthalmol Soc. 2005; 46:1586–91.
24. Kang SM, Park YS, Lee BR. The clear oil-drop residue in the vitreous cavity after intravitreal triamcinolone injection. J Korean Ophthalmol Soc. 2007; 48:665–70.
25. Lim HW, Ko BW, Song Y, et al. The clear oil-drop residue after intravitreal injection: Comparison between different brands of triamcinolone acetonide. J Korean Ophthalmol Soc. 2008; 49:1087–93.

26. Park HJ, Park JM, Oum BS. Presumed noninfectious endophalmitis after intravitreal injection of triamcinolone acetonide. J Korean Ophthalmol Soc. 2005; 46:1419–23.
27. Suh W, Lee SY, Lee JH. Clinical manifestations of presumed sterile endophalmitis after intravitreal triamcinolone injection. J Korean Ophthalmol Soc. 2008; 49:464–70.
28. Sohn HJ, Nam DH. Infectious endophthalmitis after intravitreal injection of triamcinolone acetonide. J Korean Ophthalmol Soc. 2006; 47:1865–70.
29. Shin JH, Kim DK, Yu SY, Kwak HW. A case of central retinal artery occlusion after intravitreal triamcinolone acetonide injection for diabetic macular edema in non-proliferative diabetic retinopathy. J Korean Ophthalmol Soc. 2006; 47:667–71.
30. Song HJ, Sohn HJ, Lee DY, Nam DH. Tractional retinal detachment after intravitreal bevacizumab(Avastin®) injection in proliferative diabetic retinopathy. J Korean Ophthalmol Soc. 2009; 50:1751–4.
31. Floman N, Zor U. Mechanism of steroid action in ocular inflammation: inhibition of prostaglandin production. Invest Ophthalmol Vis Sci. 1977; 16:69–73.
32. Wilson CA, Berkowitz BA, Sato Y, et al. Treatment with intravitreal steroid reduces blood-retinal barrier breakdown due to retinal photocoagulation. Arch Ophthalmol. 1992; 110:1155–9.

33. Cardillo JA, Melo LA Jr, Costa RA, et al. Comparison of intravitreal versus posterior sub-Tenon'’s capsule injection of triamcinolone acetonide for diffuse diabetic macular edema. Ophthalmology. 2005; 112:1557–63.

34. Inoue M, Takeda K, Morita K, et al. Vitreous concentrations of triamcinolone acetonide in human eyes after intravitreal or subtenon injection. Am J Ophthalmol. 2004; 138:1046–8.

35. Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994; 118:445–50.

36. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331:1480–7.

37. Paccola L, Costa RA, Folgosa MS, et al. Intravitreal triamcinolone versus bevacizumab for treatment of refractory diabetic macular edema(IBEME study). Br J Ophthalmol. 2008; 92:76–80.
38. Shimura M, Nakazawa T, Yasuda K, et al. Comparative therapy evaluation of intravitreal bevacizumab and triamcinolone acetonide on persistent diffuse diabetic macular edema. Am J Ophthalmol. 2008; 145:854–61.

39. Giles CL. Bulbar perforation during periocular injection of corticosteroids. Am J Ophthalmol. 1974; 77:438–41.

40. McLean EB. Inadvertent injection of corticosteroid into the choroidal vasculature. Am J Ophthalmol. 1975; 80:835–7.

41. O’Connor GR. Periocular corticosteroid injections: uses and abuses. Eye Ear Nose Throat Mon. 1976; 55:83–8.
42. Pieramici DJ, Avery RL, Castellarin AA, et al. Case of anterior uveitis after intravitreal injection of bevacizumab. Retina. 2006; 26:841–2.

43. Meyer CH, Mennel S, Schmidt JC, Kroll P. Acute retinal pigment epithelial tear following intravitreal bevacizumab (Avastin) injection for occult choroidal neovascularization seconday to age related macular degeneration. Br J Ophthalmol. 2006; 90:1207–8.
Go to : 
 | Figure 1.Diagram showing the pooled summary estimates of visual acuity (logarithm of the minimum angle of resolution units). IVTA=intravitreal triamcinolone acetonide injection; STTA=sub-tenon’s triamcinolone acetonide injection; CI=confidence interval; Chi2=chi-square statistic; df=degrees of freedom; P=p value; I2=I-square heterogeneity statistic; Z=Z-statistic. |
 | Figure 2.Diagram showing the pooled summary estimates of central macular thickness (micrometers). IVTA=intravitreal triamcinolone acetonide injection; STTA=sub-tenon’s triamcinolone acetonide injection; CI=confidence interval; Chi2=chi-square statistic; df=degrees of freedom; P=p value; I2=I-square heterogeneity statistic; Z=Z-statistic. |
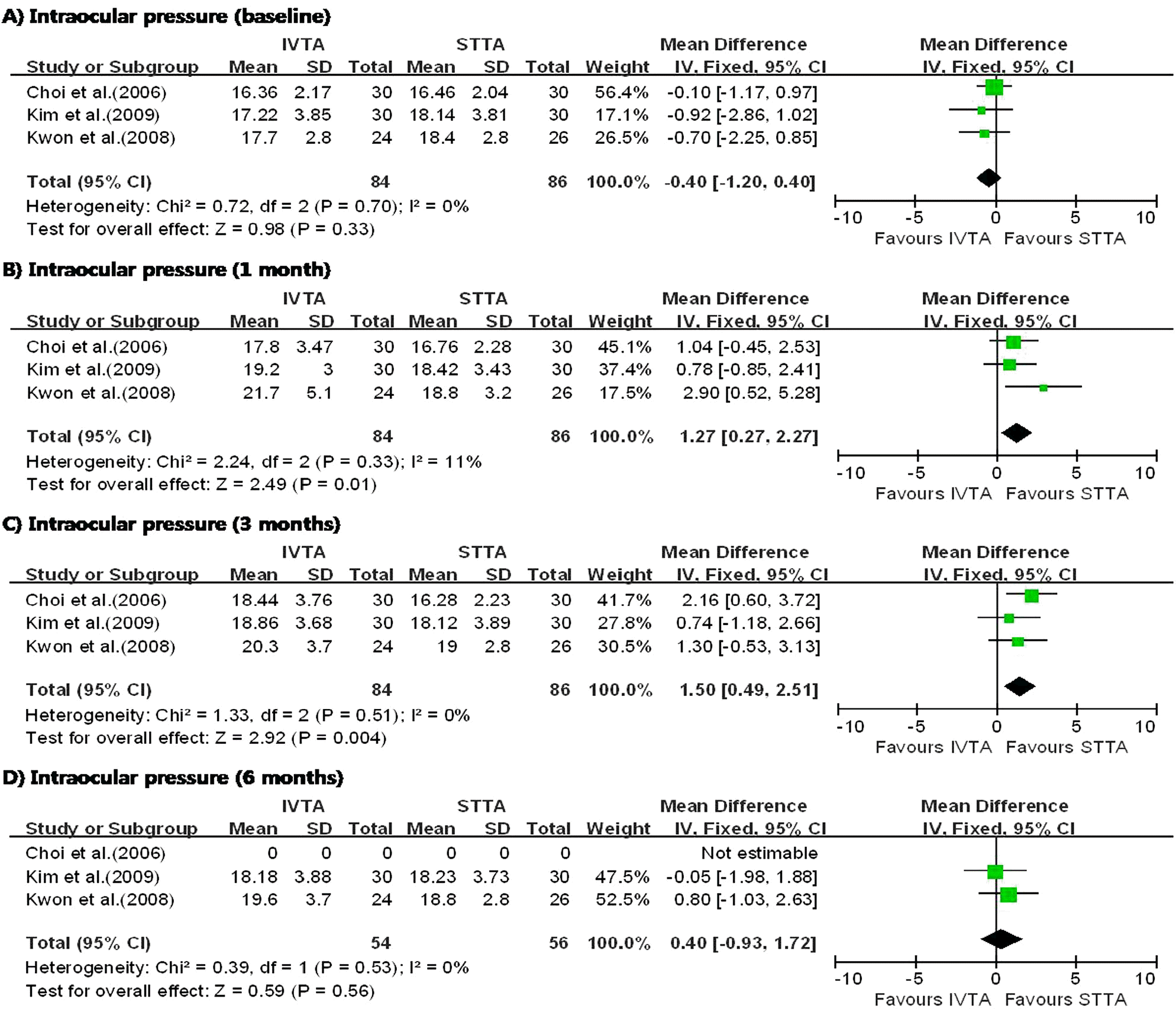 | Figure 3.Diagram showing the pooled summary estimates of intraocular pressure (millimeters of mercury). IVTA= intravitreal triamcinolone acetonide injection; STTA=sub-tenon’s triamcinolone acetonide injection; CI=confidence interval; Chi2=chi-square statistic; df=degrees of freedom; P=p value; I2=I-square heterogeneity statistic; Z=Z-statistic. |
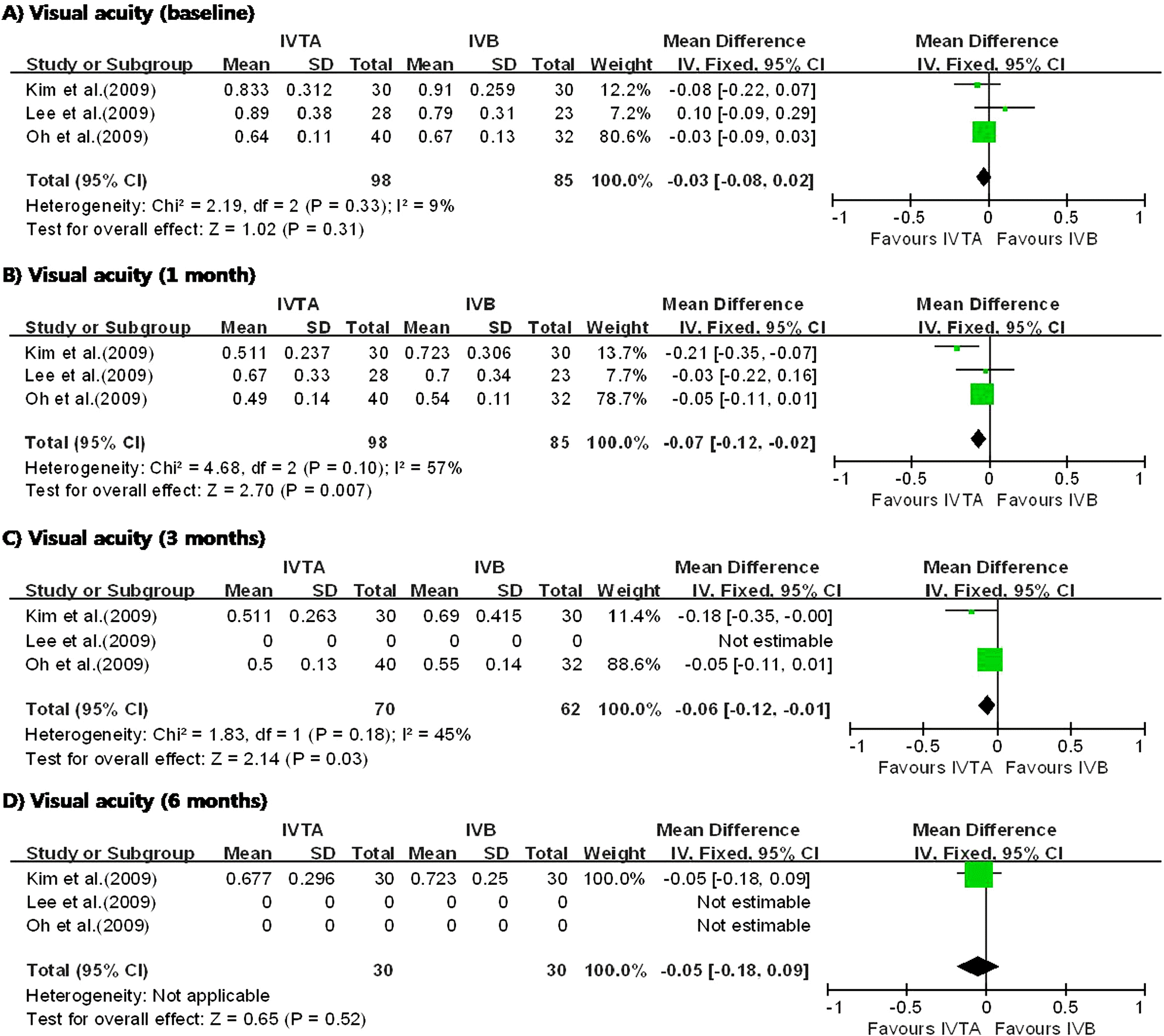 | Figure 4.Diagram showing the pooled summary estimates of visual acuity (logarithm of the minimum angle of resolution units). IVTA=intravitreal triamcinolone acetonide injection; IVB=intravitreal bevacizumab injection; CI=confidence interval; Chi2=chi-square statistic; df=degrees of freedom; P=p value; I2=I-square heterogeneity statistic; Z=Z-statistic. |
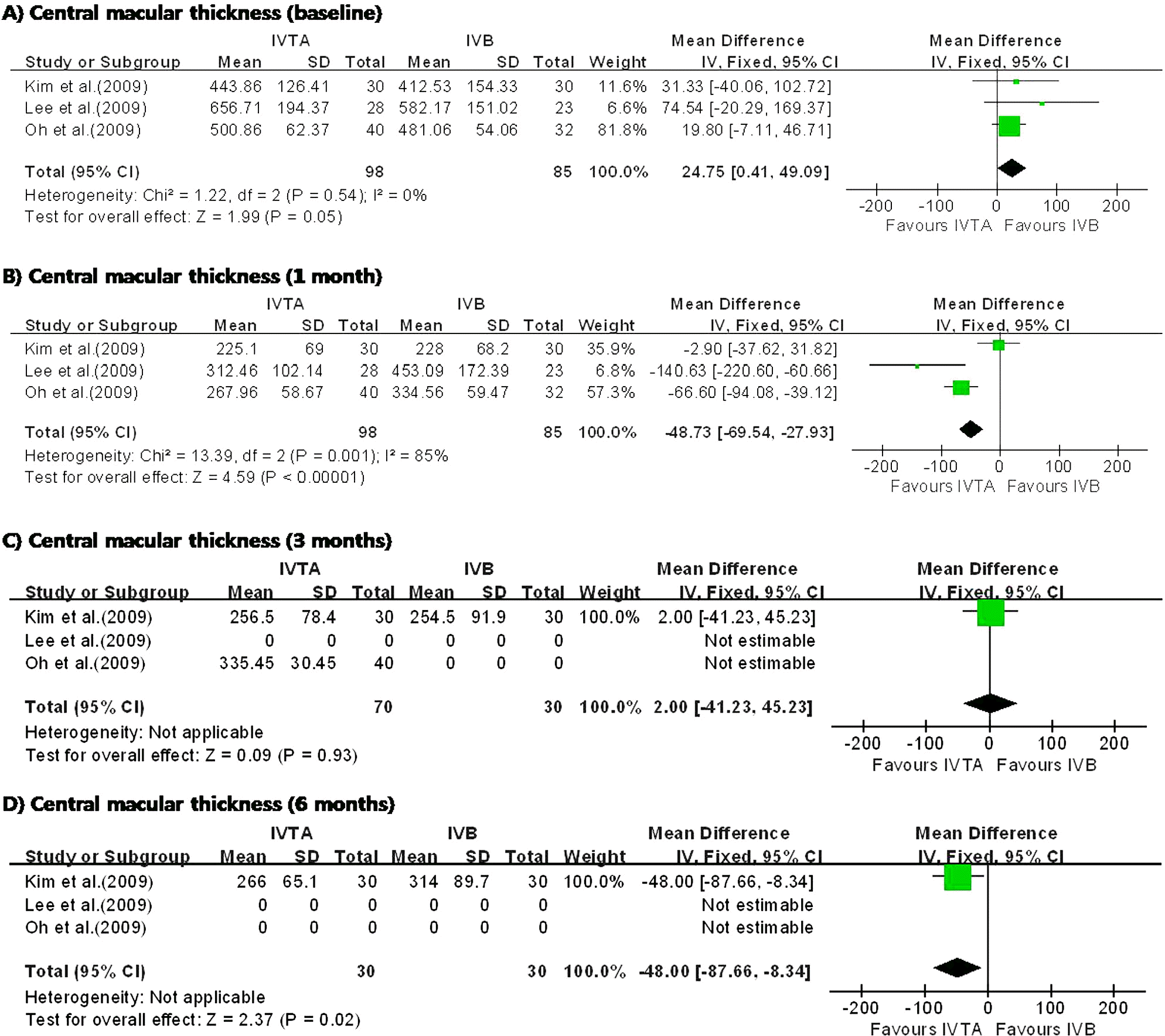 | Figure 5.Diagram showing the pooled summary estimates of central macular thickness (micrometers). IVTA=intravitreal triamcinolone acetonide injection; IVB=intravitreal bevacizumab injection; CI=confidence interval; Chi2= chi-square statistic; df=degrees of freedom; P=p value; I2=I-square heterogeneity statistic; Z=Z-statistic. |
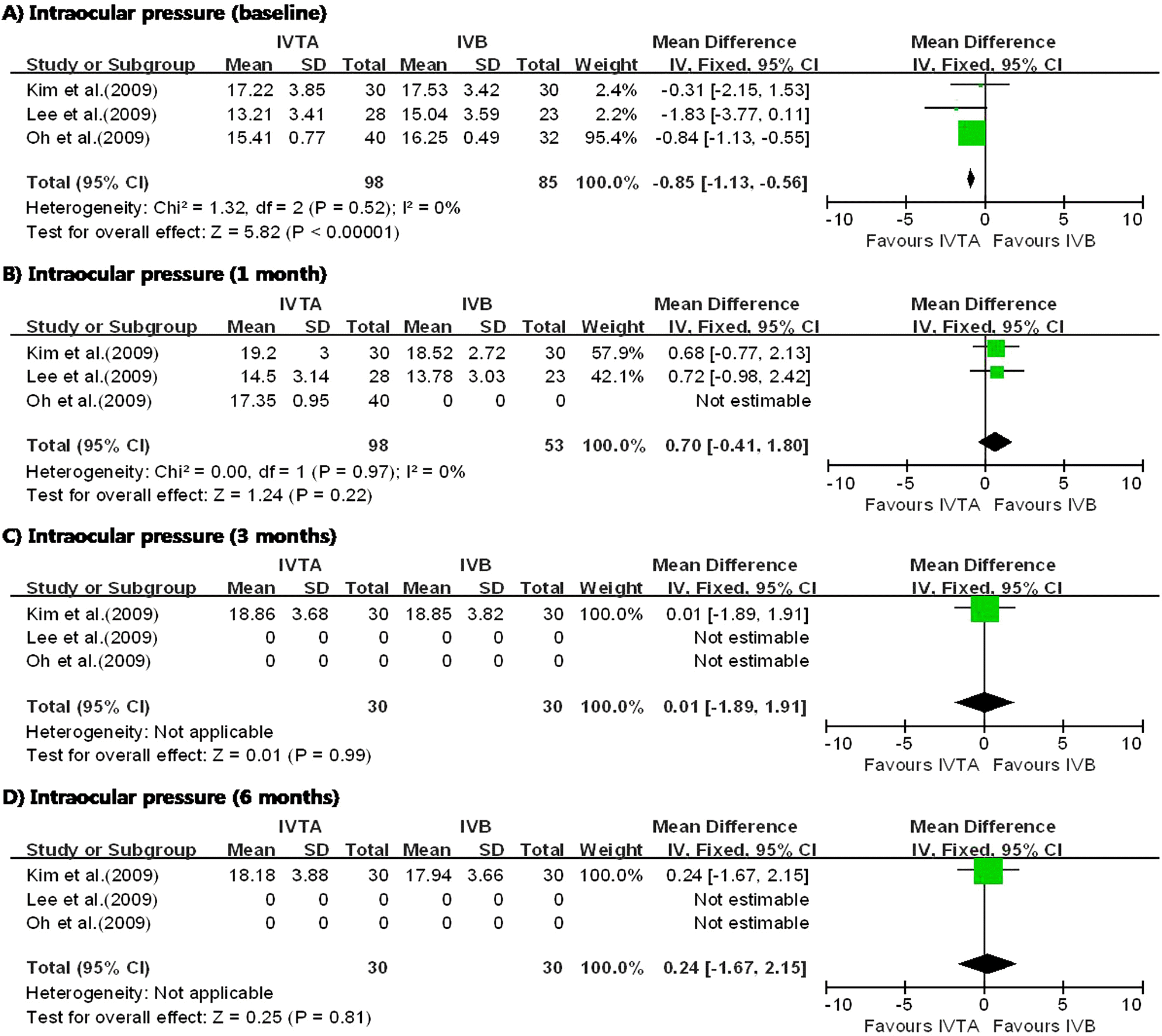 | Figure 6.Diagram showing the pooled summary estimates of intraocular pressure (millimeters of mercury). IVTA= intravitreal triamcinolone acetonide injection; IVB=intravitreal bevacizumab injection; CI=confidence interval; Chi2=chi-square statistic; df=degrees of freedom; P=p value; I2=I-square heterogeneity statistic; Z=Z-statistic. |
Table 1.
Characteristics of the 7 included studies
| Author (year) | Exposure | Dose | Patient (n) | Eye (n) | Mean age (years) | Sex (%, male) |
|---|---|---|---|---|---|---|
| Choi et al (2006)14 | IVTA* | 4 mg | 60 | 30 | 60.7 | 33.3% |
| STTA† | 40 mg | 30 | ||||
| Hwang et al (2007)18 | IVTA | 4 mg | 29 | 15 | 59.6 | 65.5% |
| IVTA+STTA | 4 mg+20 mg | 14 | ||||
| Kwon et al (2008)15 | IVTA | 4 mg | 43 | 24 | 58.9 | 41.9% |
| STTA | 40 mg | 26 | ||||
| Chang et al (2008)19 | IVTA | 4 mg | 69 | 45 | 57.0 | 50% |
| IVTA+IVB‡ | 2 mg+1.25 mg | 24 | ||||
| Lee et al (2009)16 | IVTA | 4 mg | 51 | 28 | 61.4 | 47.1% |
| IVB | 1.25 mg | 23 | ||||
| Oh et al (2009)17 | IVTA | 4 mg | 72 | 40 | 60.1 | 61.1% |
| IVB | 1.25 mg | 32 | ||||
| Kim et al (2009)13 | IVTA | 4 mg | 113 | 30 | 60.5 | 54% |
| STTA | 40 mg | 30 | ||||
| IVB | 1.25 mg | 30 | ||||
| IVB+STTA | 1.25 mg+40 mg | 30 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download