Abstract
Purpose
To evaluate long-term effects and usefulness of combined intravitreal injection of bevacizumab and panretinal photocoagulation (PRP) in patients with high-risk proliferative diabetic retinopathy.
Methods
The authors retrospectively reviewed the records of 40 patients (40 eyes) with high-risk proliferative diabetic retinopathy who had been treated with PRP alone (laser treatment group, n=20) or intravitreal bevacizumab before PRP (combined treatment group, n=20). Changes in best corrected visual acuity (BCVA), central macular thickness (CMT) and the total area of leakage from active new vessels (NVs) were compared between the groups at one, three, and six months and at one year post-treatment.
Results
In the combined treatment group, CMT decreased significantly at one month (p=0.021), and the areas of active NVs decreased significantly at one month (p=0.001) and three months (p=0.014) compared to those of the laser treatment group. However, there were no differences between the two groups after three months. In the combined treatment group, elevated intraocular pressures were found in three cases after one month, and there were vitreous hemorrhages in two cases after three months.
Go to : 
References
1. Klein R. Retinopathy in a population-based study. Trans Am Ophthalmol Soc. 1992; 90:561–94.
2. Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS Report No.12. Ophthalmology. 1991; 98:823–33.
3. Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy: clinical application of Diabetic Retinopathy Study (DRS): DRS report No. 8. Ophthalmology. 1981; 88:583–600.
4. Flynn HW Jr, Chew EY, Simons BD, et al. Pars plana vitrectomy in the Early Treatment Diabetic Retinopathy Study. ETDRS Report No. 17. Ophthalmology. 1992; 99:1351–7.
5. Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS Report No. 9. Ophthalmology. 1991; 98:766–85.
6. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331:1480–7.

7. Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994; 118:445–50.

8. Adamis AP, Shima DT, Tolentino MJ, et al. Inhibition of vascular endothelial growth factor prevents retinal ischaemia associated iris neo-vascularization in a non-human primate. Arch Ophthalmol. 1996; 114:66–71.
9. Cunningham ET Jr, Adamis AP, Altaweel M, et al. A phase II randomized doublemasked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005; 112:1747–57.

10. Chun DW, Heier JS, Topping TM, Duker JS & Bankert JM. Pilot study of multiple intravitreal injections of ranibizumab in patients with centre-involving clinically significant diabetic macular edema. Ophthalmology. 2006; 113:1707–12.
11. Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina. 2006; 26:352–4.

12. Spaide RF & Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous haemorrhage. Retina. 2006; 26:275–8.
13. Jorge R, Costa RA, Calucci D, et al. Intravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE Study). Retina. 2006; 26:1006–13.

14. Cho WB, Oh SB, Moon JW, et al. Panretinal photocoagulation combined with intravitreal bevacizumab in high-risk proliferative diabetic retinopathy. Retina. 2009; 29:516–22.

15. Chang JK, Chang MH. Therapeutic Effects of Intravitreal Bevacizumab Injection for Retinal Neovascularization Secondary to Proliferative Diabetic Retinopathy. J Korean Ophthalmol Soc. 2009; 50:1359–70.

16. McDonald HR, Schatz H. Macular edema following panretinal photocoagulation. Retina. 1985; 5:5–10.

17. McDonald HR, Schatz H. Visual loss following panretinal photo-coagulation for proliferative diabetic retinopathy. Ophthalmology. 1985; 92:388–93.

18. Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology. 1991; 98:766–85.
19. Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002; 109:920–7.

20. Choi KS, Chung JK, Lim SH. Laser Photocoagulation Combined with Intravitreal Triamcinolone Acetonide Injection in Proliferative Diabetic Retinopathy with Macular Edema. Korean J Ophthalmology. 2007; 21:11–7.

21. Jonas JB, Degenring R, Kreissig I, et al. Safety ofintravitreal high-dose reinjections of triamcinolone acetonide. Am J Ophthalmol. 2004; 138:1054–5.
22. Gillies MC, Simpson JM, Billson FA, et al. Safety of an intravitreal injection of triamcinolone: results from a randomized clinical trial. Arch Ophthalmol. 2004; 122:336–40.
23. Nonaka A, Kiryu J, Tsujikawa A, et al. Inflammatory response after scatter laser photocoagulation in nonphotocoagulated retina. Invest Ophthalmol Vis Sci. 2002; 43:1204–9.
24. Brooks HL Jr, Caballero S Jr, Newell CK, et al. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch Ophthalmol. 2004; 122:1801–7.
25. Haritoglou C, Kook D, Neubauer A, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006; 26:999–1005.

26. Wu L, Martínez-Castellanos MA, Quiroz-Mercado H, et al. For the Pan American Collaborative Retina Group (PACORES). Twelve-month safety of intravitreal injections of bevacizumab: Results of the PACORES. Graefes Arch Clin Exp Ophthalmol. 2008; 246:81–7.
Go to : 
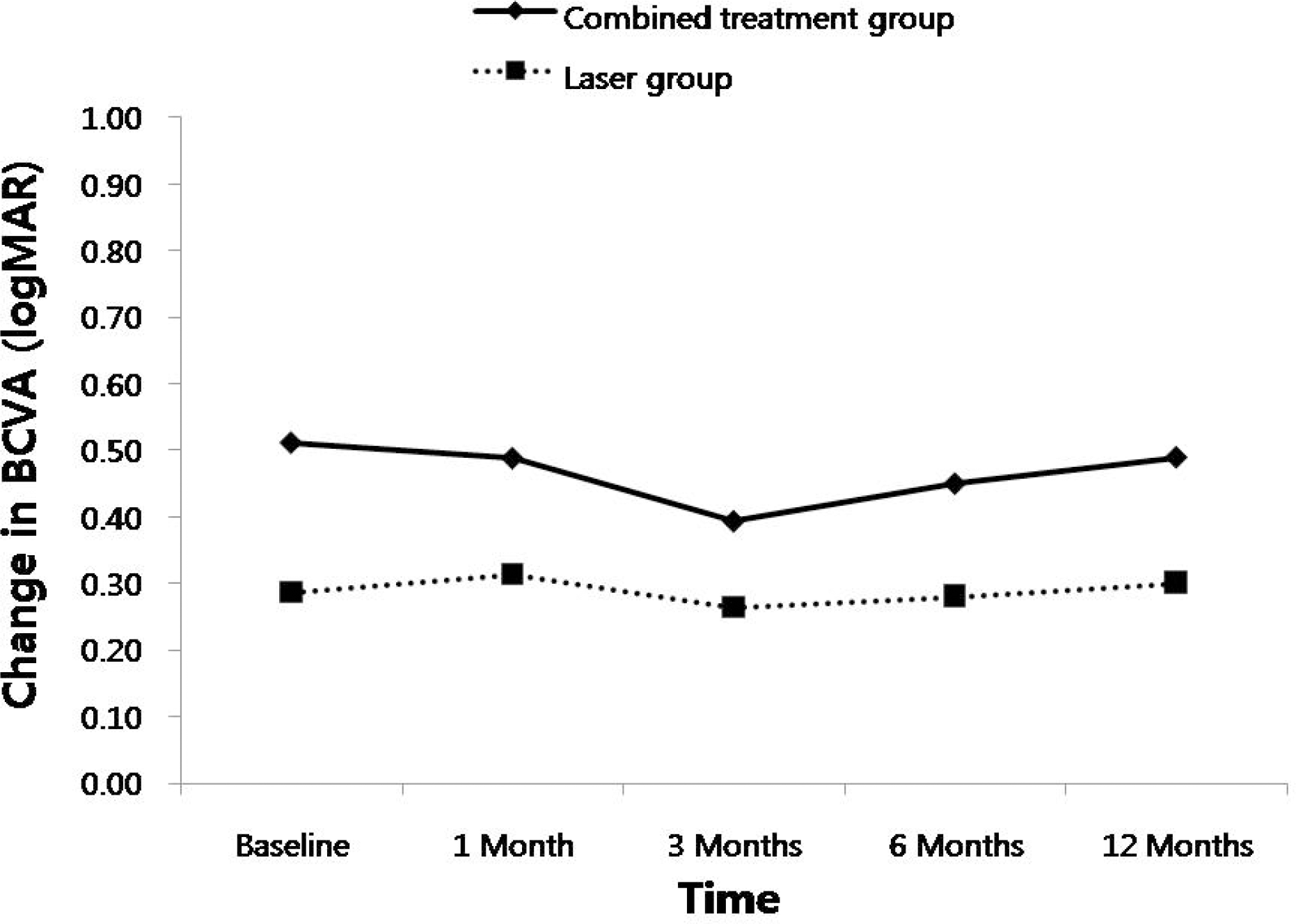 | Figure 1.Changes in best corrected visual acuity (BCVA) after treatments in the two groups. There was no significant difference of changes in IOP between the groups at each visit. logMAR=logarithm of minimum angle of resolution. |
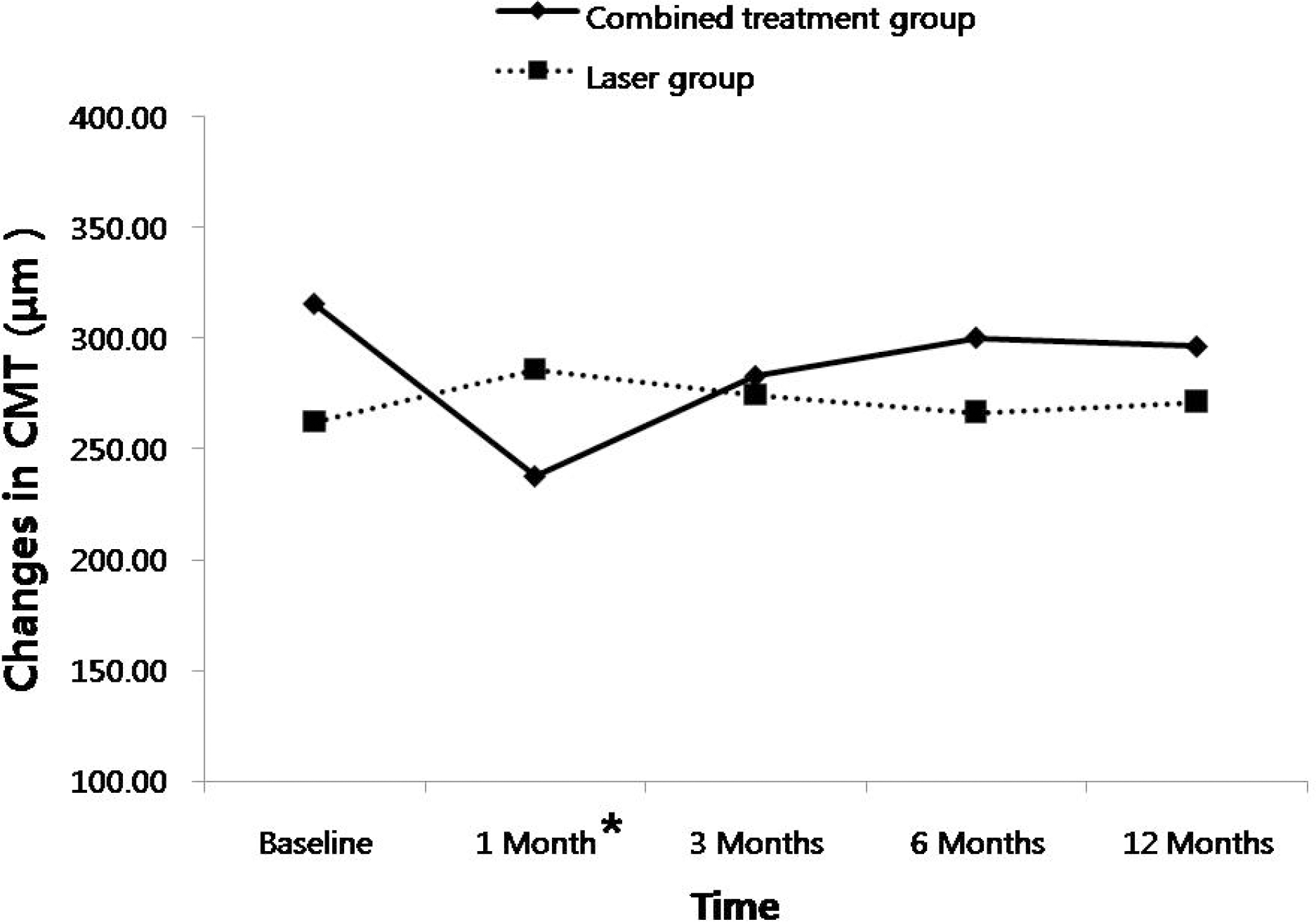 | Figure 2.Changes in central macular thickness (CMT) after treatments in the two groups. Combined treatment group revealed significant reduction of CMT compared to laser treatment group at 1 month after treatment (p=0.021). There was no difference between the groups at the other visits. * Statistically significant differences between the two groups |
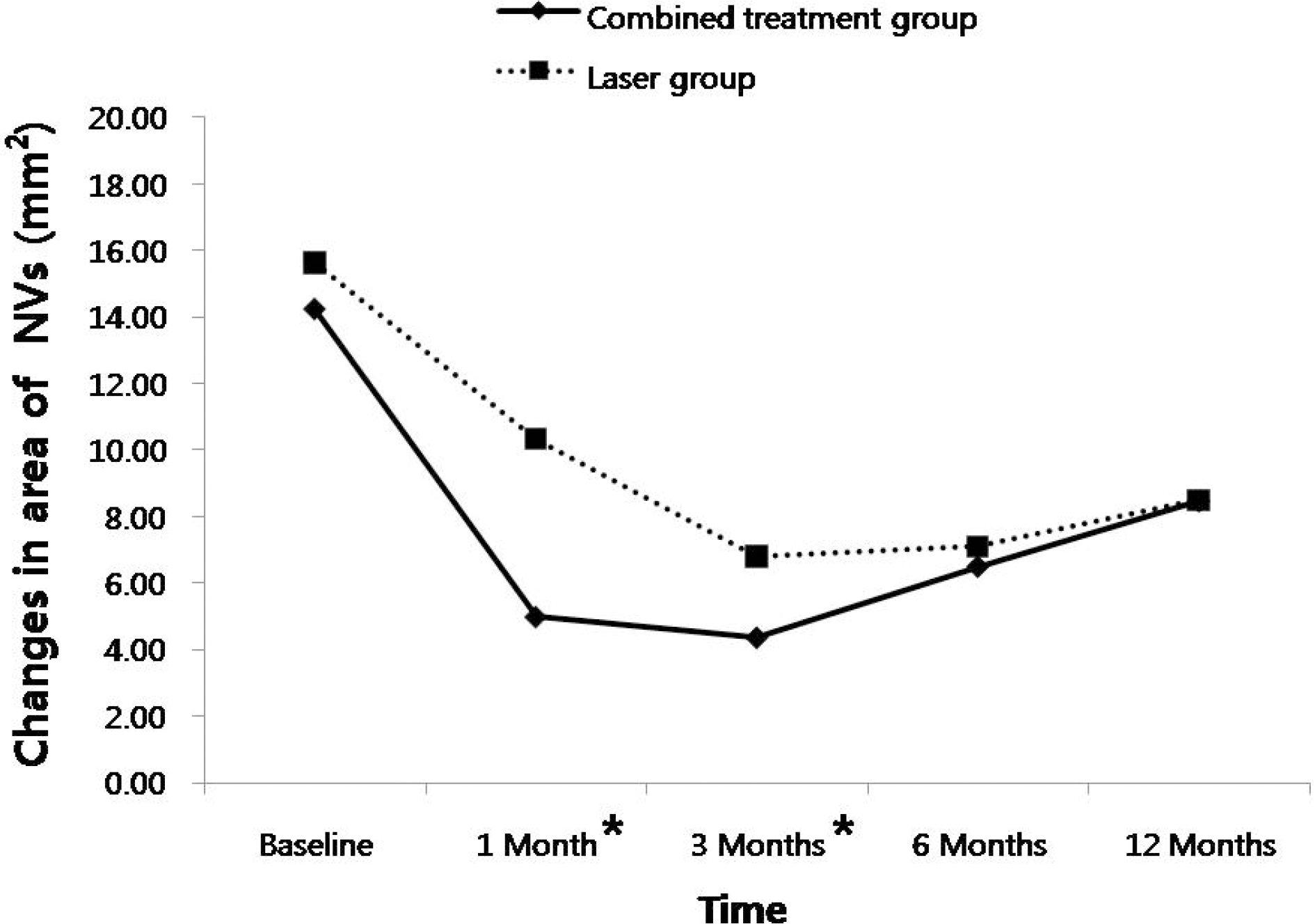 | Figure 3.Changes in the total area of leakage from active new vessels (NVs) after treatments in the two groups. Combined treatment group revealed significant reduction in the area of active NVs compared to laser treatment group at 1 month (p=0.001) and 3 months (p=0.014) after treatment. However, There was no difference among groups at 6 months and 12 months * Statistically significant differences after treatment. between the two groups. |
Table 1.
Baseline characteristics of two groups enrolled in the study
| Characteristics | Combined treatment group | Laser treatment group | P value |
|---|---|---|---|
| (20 eyes) | (20 eyes) | ||
| Age, years (Mean± SD) | 53.5±10.5 | 55.7±12.1 | 0.643* |
| Gender, n (Male : Female) | 9 : 11 | 10 : 10 | 0.752† |
| Duration of DM, years (Mean± SD) | 10.5±6.9 | 9.6±4.8 | 0.896* |
| HbA1c, % (Mean± SD) | 7.43±2.1 | 7.25±2.02 | 0.835* |
| Insulin treatment, n | 15 (75%) | 12 (60%) | 0.311† |
| Hypertension, n | 8 (40%) | 11 (55%) | 0.342† |
| CSME, n | 11 (55%) | 8 (40%) | 0.342† |
| Initial BCVA (logMAR) | 0.51±0.49 | 0.29±0.31 | 0.224* |
| Initial CMT, μm (Mean± SD) | 315.6±94.1 | 262.3±50.7 | 0.100* |
| Initial area of NVs, mm2 (Mean± SD) | 14.2±4.9 | 15.6±5.2 | 0.737* |
Table 2.
Best corrected visual acuity (BCVA), central macular thickness (CMT) and the total area of leakage from active new vessels (NVs) in the combined treatment group at each visit
| Study period | BCVA | CMT | Area of NVs |
|---|---|---|---|
| (logMAR, Mean± SD) | (μm, Mean± SD) | (mm2, Mean± SD) | |
| Baseline | 0.51±0.49 | 315.6±94.1 | 14.23±4.83 |
| 1 Month | 0.49±0.46 | 237.9±74.8* | 4.99±3.62* |
| 3 Months | 0.39±0.40* | 283.0±75.3* | 4.37±2.74* |
| 6 Months | 0.45±0.42 | 300.3±86.2 | 6.50±3.48* |
| 12 Months | 0.49±0.41 | 296.4±86.8 | 8.47±3.71* |
Table 3.
Best corrected visual acuity (BCVA), central macular thickness (CMT) and the total area of leakage from active new vessels (NVs) in the laser treatment group at each visit
| Study period | BCVA | CMT | Area of NVs |
|---|---|---|---|
| (logMAR, Mean± SD) | (μm, Mean± SD) | (mm2, Mean± SD) | |
| Baseline | 0.29±0.13 | 262.3±50.1 | 15.64±5.20 |
| 1 Month | 0.31±0.15 | 285.8±46.2* | 10.33±3.62* |
| 3 Months | 0.26±0.13 | 274.5±45.1 | 6.79±3.74* |
| 6 Months | 0.28±0.14 | 266.6±71.6 | 7.09±3.43* |
| 12 Months | 0.30±0.17 | 271.3±58.9 | 8.89±2.98* |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download