Abstract
Purpose
To investigate the biologic effects of topical ocular artificial tears used in patients wearing contact lens on in vitro corneal epithelial cells.
Methods
The efficacies of the topical artificial tears Iris®, Irisplus®, Eyemiru contact pure®, and Eye2O® were evaluated using the MTT and wound healing assays. Cell damage was determined using lactate dehydrogenase (LDH), and the solution ingredients were analyzed. Cellular morphologies were examined by inverted light microscopy and transmission electromicroscopy.
Results
Metabolic activity of corneal epithelial cells, as determined by the MTT assay, decreased in the Iris® eye drop group, but those of the other groups were similar to that of the control. The LDH titers increased up to one hour after Iris® eye drop use, and the increased level was maintained for 24 hours. The other three artificial tears showed similar low LDH titers to that of the control. Cellular migration was not observed, although cellular damage to the corneal epithelial cells, such as chromatin margination and cytoplasmic organelle swelling, was prominent with Iris® use.
Go to : 
References
1. Businger U, Treiber A, Flury C. The etiology and management of three and nine o'clock staining. Int Contact Lens Clin. 1989; 16:136–9.

2. Foulks GN. What is dry eye and what does it mean to the contact lens wearer? Eye Contact Lens. 2003; 29:S96–100.

3. Orsborn GN, Zantos SG. Practitioner survey: management of dry-eye symptoms in soft lens wearers. Contact Lens Spectrum. 1989; 4:23–6.
4. Solomon J. Causes and treatments of peripheral corneal desiccation. Contact Lens Forum. 1986; 11:30–6.
5. Reuben M, Watkins R. Pilocarpine dispensation for the soft abdominal contact lens. Br J Ophthalmol. 1975; 59:455–8.
6. Jain MR. Drug delivery though soft contact lenses. Br J Ophthalmol. 1988; 72:150–4.
7. Silbert JA. A review of therapeutic agents and contact lens wear. J Am Opt Asse. 1996; 67:165–72.
8. Korean Contact Lens Society. Contact Lens: Principles and Practice. 2nd ed.Vol. 1. Seoul: Nae-Oi publishing & printing;2007. p. 187–93.
9. Berdy GJ, Abelson MB, Smith LM, George MA. Preservative-free artificial tear preparations Assessment of corneal epithelial toxic effects. Arch Ophthalmol. 1992; 110:528–32.
10. Diebold Y, Herreras JM, Callejo S. Carbomer-versus cellulose-based artificial-tear formulations: morphologic and toxicologic abdominals on a corneal cell line. Cornea. 1998; 17:433–40.
11. Dutot M, Warnet JM, Baudouin C, Rat P. Cytotoxicity of contact lens multipurpose solutions: Role of oxidative stress, abdominal activity and P2X7 cell death receptor activation. Eur J Pharm Sci. 2008; 33:138–45.
12. Wee WR, Wang XW, McDonnell PJ. Effect of artificial tears on cultured keratocytes in vitro. Cornea. 1995; 14:273–9.

13. Park YS. Physiology of body fluid. Kang DH, editor. Physiology. 5th ed.Seoul: Sin-Kwang publishing & printing;2000. chap. 5.
14. Lee JS, Oum BS. The effects of artificial tear formulation and an-ti-inflammatory agents on the cultured keratocyted of rabbit. J Korean Ophthalmol Soc. 1998; 39:42–51.
15. Freshney RI. The culture environment: Substrate, gas phase, medium, and temparature. 2nd ed.New York: Wiley-Liss;1987. p. 70–1.
16. Kim K. Acid-base balance. Sung HK, Kim KH, editors. Physiology. Seoul: Eui-hak publishing & printing;1996. 7:chap. 20.
17. Burstein NL. Preservative cytotoxic threshold for benzalkonium chloride and chlorhexidine digluconate in cat and rabbit corneas. Invest Ophthalmol Vis Sci. 1980; 19:308–13.
18. Cha SH, Lee JS, Oum BS, Kim CD. Corneal epithelial cellular dysfunction from banzalkonium chloride (BAC) in vitro. Clin Exp Ophthalmol. 2004; 32:180–4.
19. Green K, Tonjum A. Influence of various agents on corneal permeability. Am J Ophthalmol. 1971; 72:897–905.

20. Lee JS, Jung DY, Oum BS, Kim CD. Cytotoxicity of abdominal chlorid on the corneal epithelial cell of rabbit. J Korean Ophthalmol Soc. 1998; 39:1326–33.
Go to : 
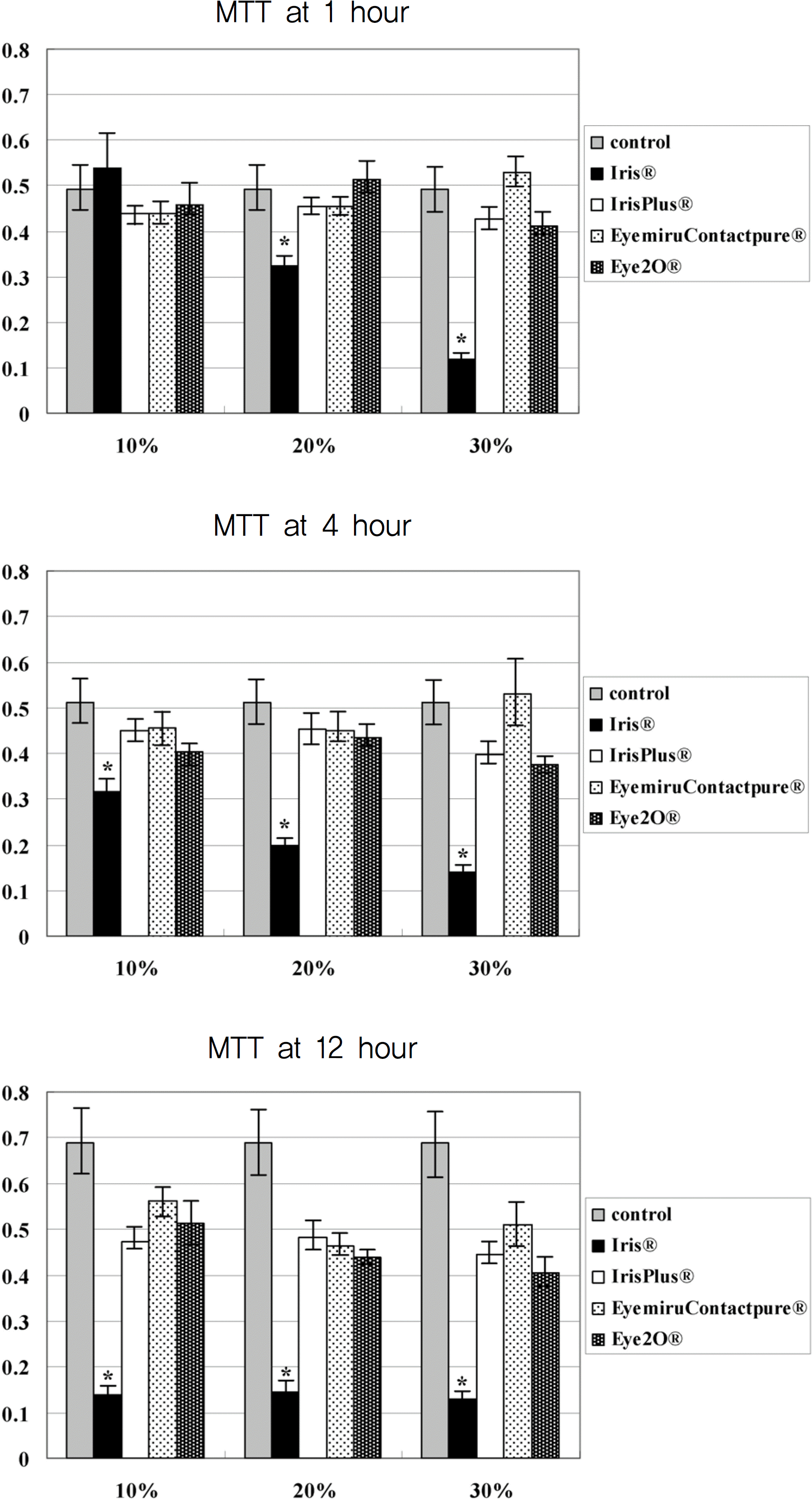 | Figure 1.The absorption rate of the water-insolubale formazan dye in corneal epithelial cell exposed in four artificial tear drops by a scanning spectrometer (ELISA reader) (* p<0.05). |
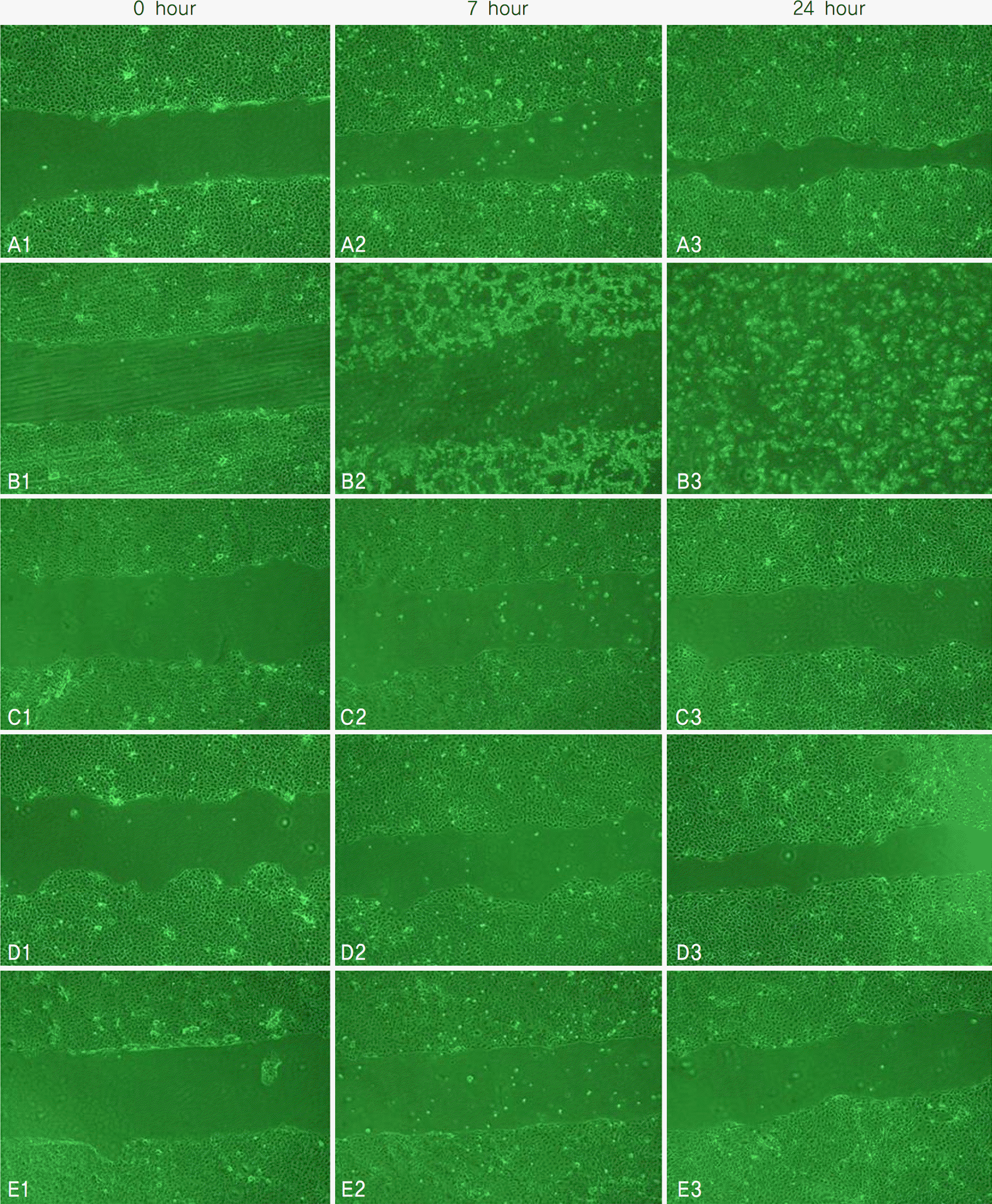 | Figure 2.Scratch assay of corneal epithelial cells after 0-hour exposure to (A1) control (B1) 20% Iris®, (C1) Irisplus®, (D1) Eyemiru contactpure®, (E1) Eye2O®, 7-hour exposure to (A2) control (B2) 20% Iris®, (C2) Irisplus®, (D2) Eyemiru contactpure®, (E2) Eye2O®, 24-hour exposure to (A3) control (B3) 20% Iris®, (C3) Irisplus®, (D3) Eyemiru contactpure® and (E3) Eye2O®. In control group (A), normal corneal epithelial cells were proliferated and moved into scratched scar in the culture medium. After exposure to Iris® (B), corneal epithelial cells were not proliferated and moved into scratched scar in the culture medium. |
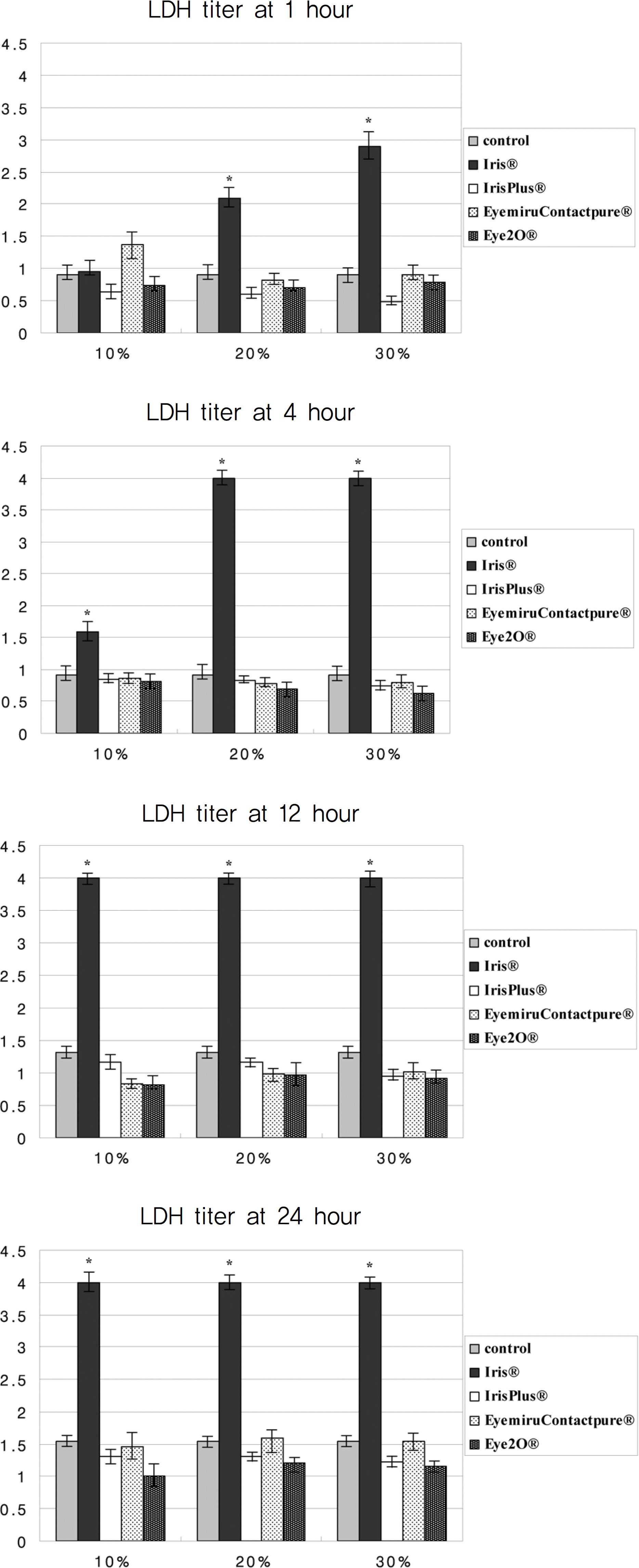 | Figure 3.LDH titers of cultured corneal epithelial cells exposed in four artificial tear drops. (* p<0.05). |
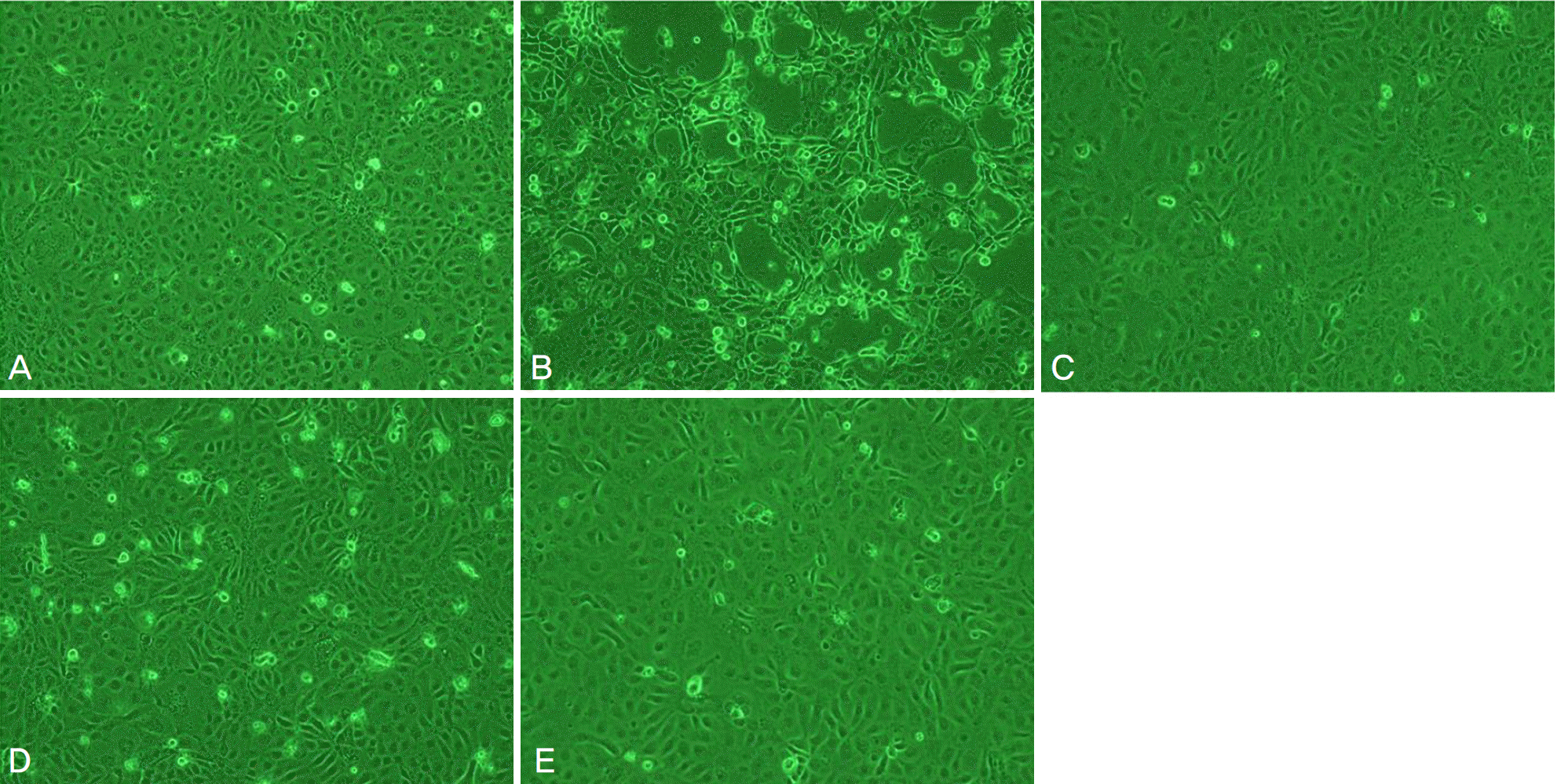 | Figure 4.Inverted light micrographs of corneal epithelial cells after 4-hour exposure to (A) control, (B) Iris®, (C) Irisplus®, (D) Eyemiru contactpure®, and (E) Eye2O®. |
 | Figure 5.Transmission electron micrographs of corneal epithelial cells appeared after 4-hour exposure to (A) control, (B) 20% Iris®, (C) 20% Irisplus®, (D) 20% Eyemiru contactpure®, and (E) 20% Eye2O®. (bar length 2 um, original magnification, ×2000∼4000). In general, the plasma membrane with microvilli (black arrow head), nuclear membrane, and nuclei of conjunctival cells were visible. Iris® had more severe and damaged cellular structures, such as the plasma membranes with microvilli being disrupted (white arrow head), well-developed vacuole formation (black arrow), and chromatin margination of the nucleus (white arrow), rather than IrisPlus®, Eyemiru contactpure®, Eye2O®. |
Table 1.
The ingredient, electrolyte composition, pH, osmolarity and preservatives of the commercial four artificial tear drops




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download