Abstract
Purpose
To identify the regression of neovascularization and relief of other symptoms after intravitreal bevacizumab and retrobulbar triamcinolone injection in neovascular glaucoma patients with no possibility of visual acuity recovery.
Methods
A total of 15 eyes in 15 patients with neovascular glaucoma, who had no possibility of visual acuity recovery and could not be treated with surgical intervention despite pain from October 2008 to May 2009, were reviewed retrospectively. Changes in degree of pain, conjunctival injection, revascularization, and visual acuity were evaluated after injection.
Results
Ten of the 15 (67%) neovascular glaucoma patients were male, with a mean age of 62.50±12.79 years. The most common prediposing ocular disease was diabetic retinopathy (9; 60%), and the others were central retinal vein occlusion (4: 26%), central retinal artery occlusion (1; 7%), and uveitis (1; 7%). Change in intraocular pressure was significant (p<0.001), as were decreases in the degrees of pain and conjunctival injection (p<0.001, <0.001) after the injections. Regression of neovascularization continued in 13 patients (87%) after two months.
References
1. Moraczewsk AL, Lee RK, Palmberg PF, et al. Outcomes of abdominal of neovascular glaucoma with intravitreal bevacizumab. Br J Ophthalmol. 2009; 93:589–93.
2. Kawaji T, Takano A, Inomata Y, et al. Trans tenon's retrobulbar triamcinolone acetonide injection for macular oedema related to branch retinal vein occlusion. Br J Ophthalmol. 2008; 92:81–3.
3. Rodriguez ML, Juarez CP, Luna JD. Intravitreal triamcinolone acetonide injection in blind painful eyes. Eur J Ophthalmol. 2003; 13:292–7.

4. Maeng H, Kim J, Kee C. Intravitreal Bevacizumab injection for the treatment of early stage neovascular glaucoma. J Korean Ophthalmol Soc. 2008; 49:696–700.
5. Allen RC, Bellows AR, Hutchinson BT, Murphy SD. Filtering surgery in the treatment of neovascular glaucoma. Ophthalmology. 1982; 89:1181–7.
6. Heuer DK, Parrish RK II, Gressel MG, et al. 5-fluorouracil and glaucoma filtering surgery. II. A Pilot study. Ophthalmology. 1984; 91:384–94.
7. Song YM, Kim JH. Photodynamic therapy with verteporfin for anterior segment neovascularization in neovascular glaucoma. J Korean Ophthalmol Soc. 2005; 46:1592–6.
8. Hayashi K, Hayashi H. Intravitreal versus retrobulbar injections of triamcinolone for macular edema associated with branch abdominall vein occlusion. Am J Ophthalmol. 2005; 139:972–82.
9. Wada M, Ogata N, Minamino K, et al. Trans tenon's retrobulbar injection of triamcinolone acetonide for diffuse diabetic macular edema. Jpn J Ophthalmol. 2005; 49:509–15.
10. Kato A, Kimura H, Okabe K, et al. Suppression of laser induced choroidal neovascularization by posterior sub tenon abdominal of triamcinolone acetonide. Retina. 2005; 25:503–9.
11. Gheith ME, Siam GA, de Barros DS, et al. Role of intravitreal abdominal in neovascular glaucoma. J Ocul Pharmacol Ther. 2007; 23:487–91.
12. Kim YH, Kim ES, Yu SY, Kwak HW. Long term effect of abdominal bevacizumab for CNV secondary to age related macular degeneration. J Korean Ophthalmol Soc. 2008; 49:1935–40.
13. Chandler DB, Rozakis G, de Juan E Jr, Machemer R. The effect of triamcinolone acetonide on a refined experimental model of abdominal vitreoretinopathy. Am J Ophthalmol. 1985; 99:686–90.
14. Schindler RH, Chandler D, Thresher R, Machemer R. The abdominal of intravitreal triamcinolone acetonide. Am J Ophthalmol. 1982; 93:415–7.
15. Mc Cuen BW II, Besslar M, Tano Y, et al. The lack of toxicity of intravitreally administrated triamcinolone acetonide. Am J Ophthalmol. 1981; 91:785–8.
16. Scholes GN, O'Brien WJ, Abrans GW, Kubicek MF. Clearance of triamcinolone from vitreous. Arch Ophthalmol. 1985; 103:1567–9.

17. Kaushik S, Gupta V, Gupta A, et al. Intractable glaucoma abdominal intravitreal triamcinolone in central retinal vein occlusion. Am J Ophthalmol. 2004; 137:758–60.
18. Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003; 136:791–6.

19. Nelson ML, Tennant MT, Sivalingam A, et al. Infectious and abdominal noninfectious endophthalmitis after intravitreal abdominal acetonide injection. Retina. 2003; 23:686–91.
20. McCartney HJ, Drysdale IO, Gornall AG, et al. An autoradio-graphic study of the penetration of subconjunctival injected hy-drocortisone into the normal and inflamed rabbit eye. Invest Ophthalmol. 1965; 4:297–302.
Figure 1.
Patient was asked to check the degree of subjective pain using the visual analogue scale at different visiting time (0; no pain, 10; Worst pain that can imagine). (A) Pre-injection time, (B) One week after injection, (C) One month after injection.
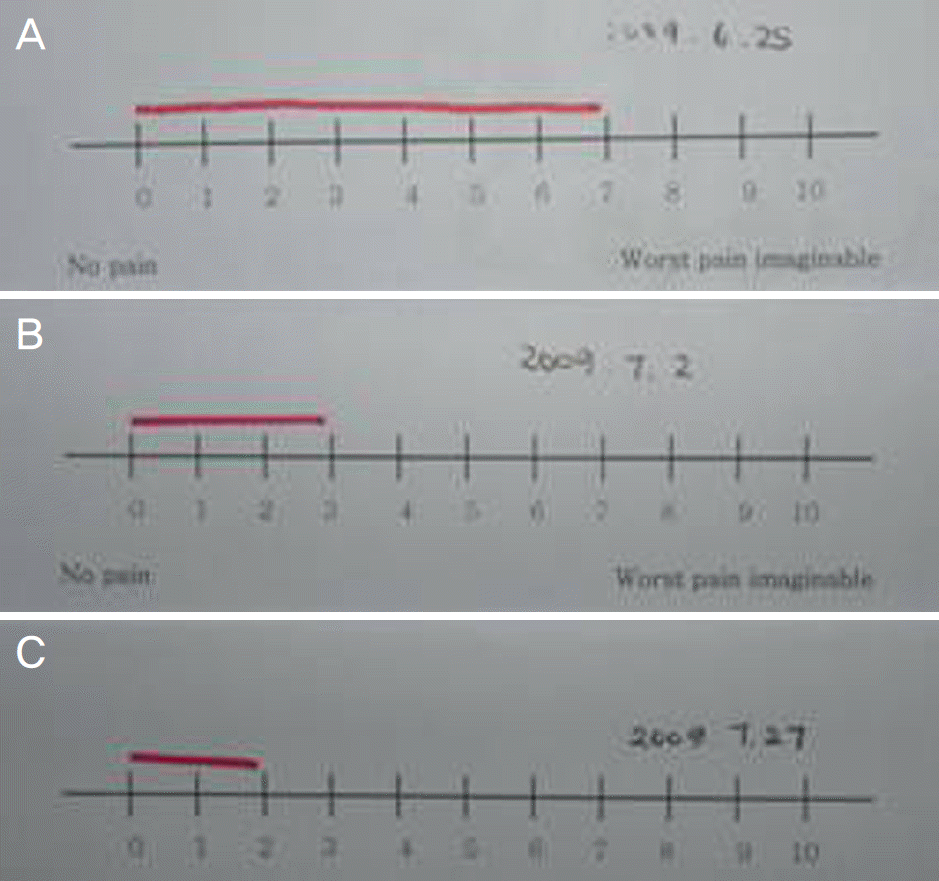
Figure 2.
Change in and grade of conjunctival injection. (A) Pre-injection state (severe conjunctiva vascular con-gestions and epithelial edema; grade 3), (B) After 1 week (grade 2), (C) After 2 months (grade 0).
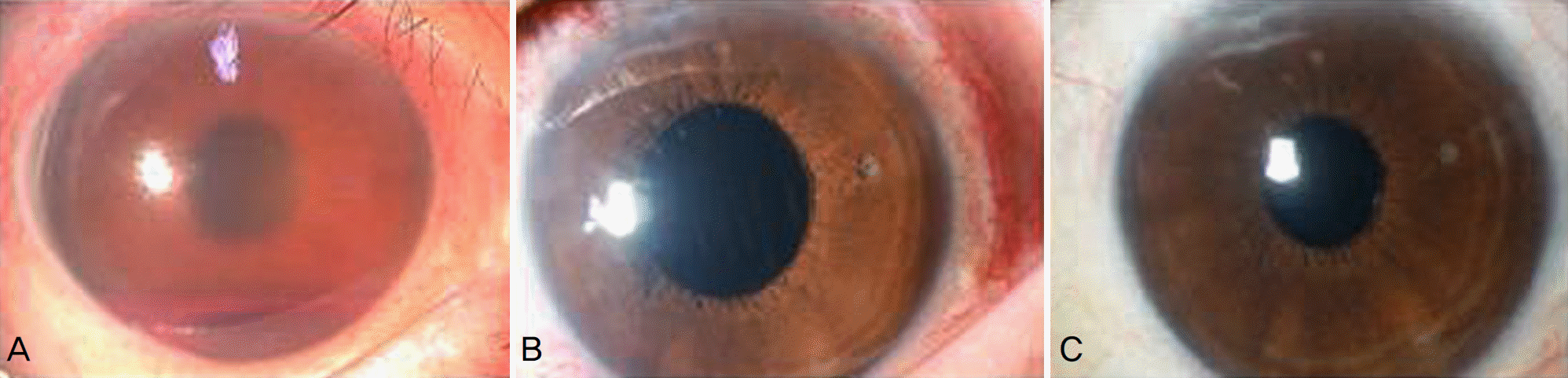
Figure 3.
Change in IOP after intravitreal bevacizumab and retrobulbar triamcinolone injection (p<0.001).
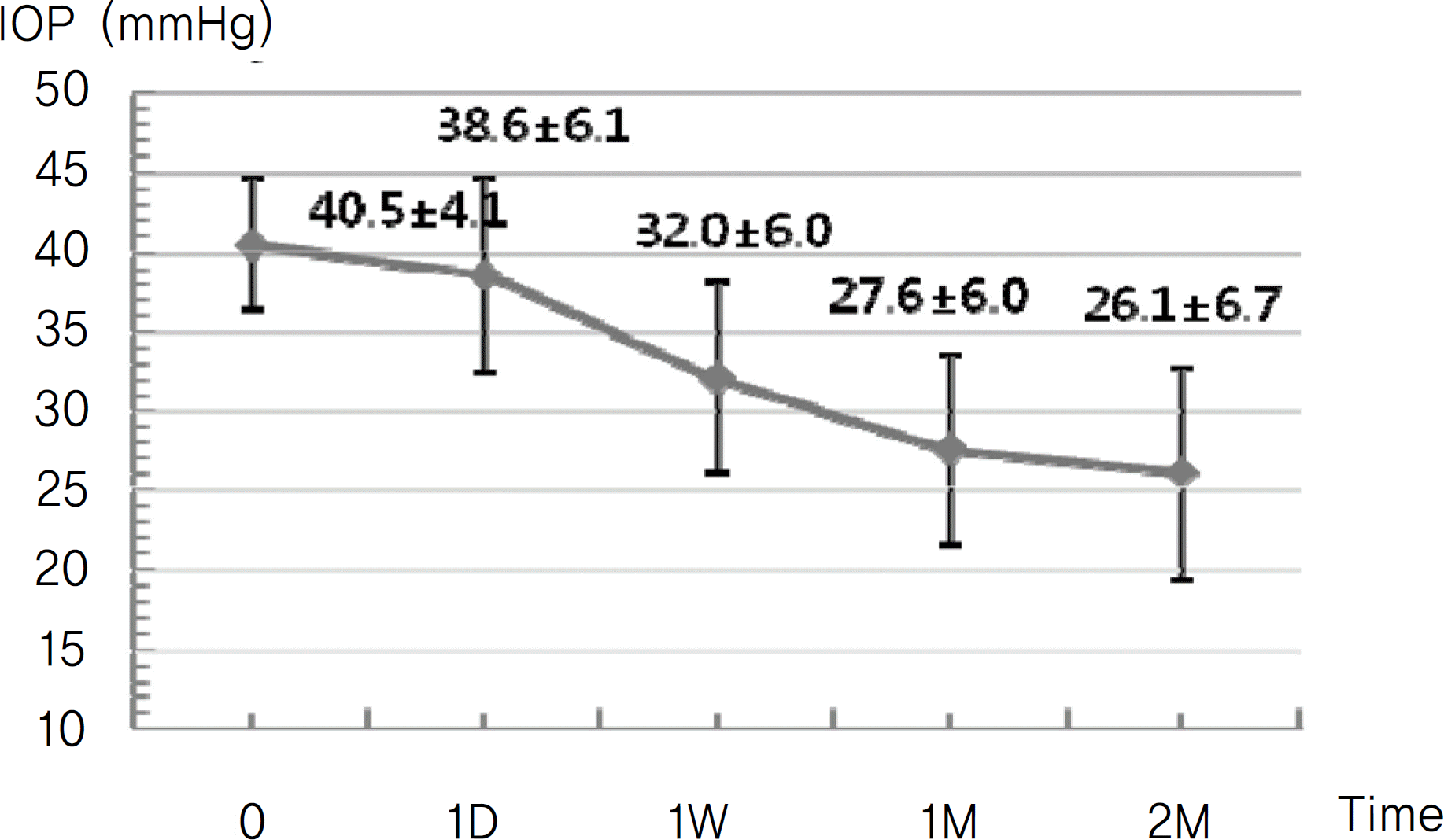
Figure 4.
Change in pain degree after intravitreal bevacizumab and retrobulbar triamcinolone injection (p<0.001).
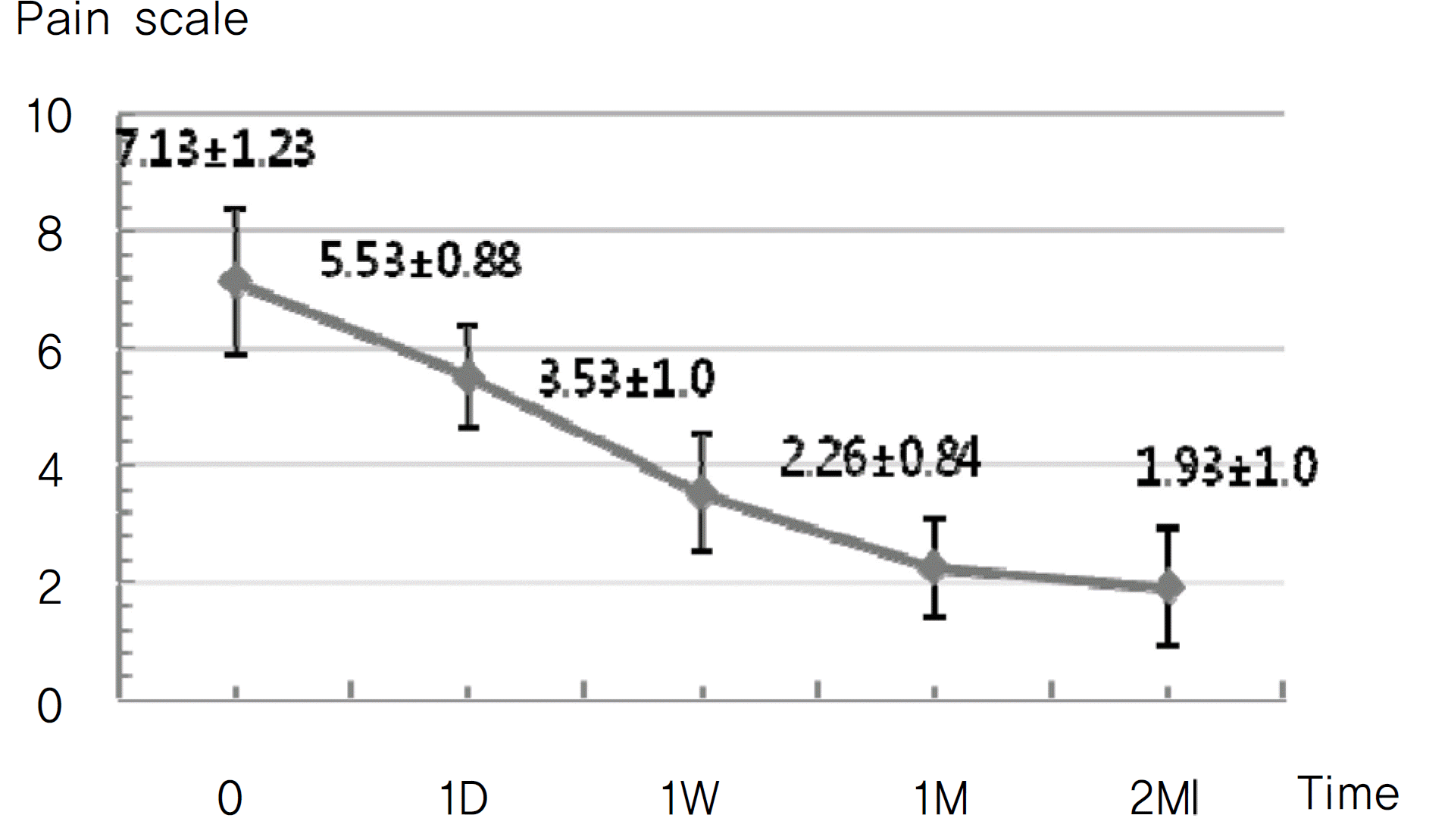
Figure 5.
Change in conjunctival injection after intravitreal bevacizumab and retrobulbar triamcinolone injection (p<0.001).
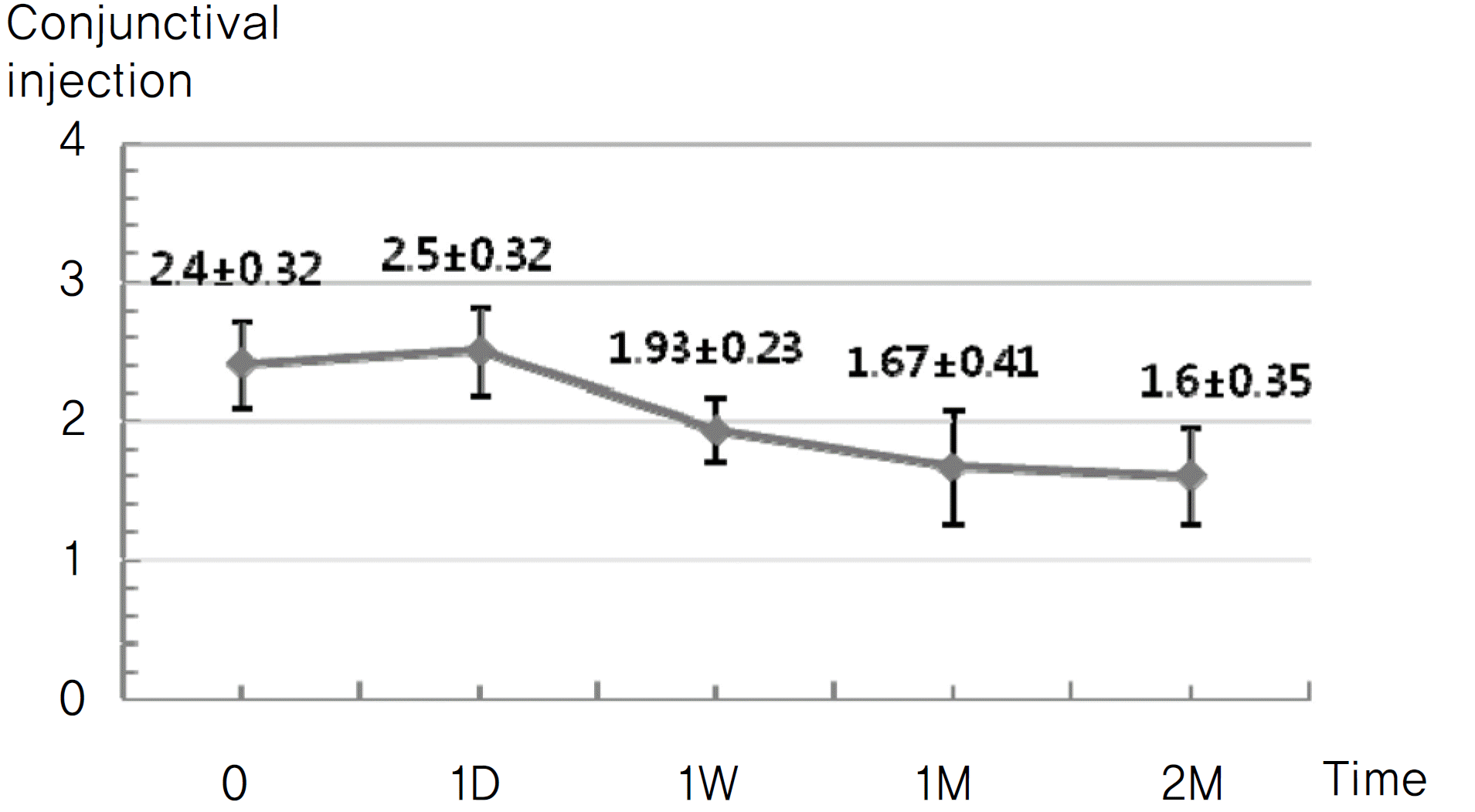
Figure 6.
Change in neovascularization. (A) Neovascularization on the pupil margin at pre-injection time. (B) Disappearance of neovascularization on the pupil margin and decreasing corneal epithelial edema after 1 week.
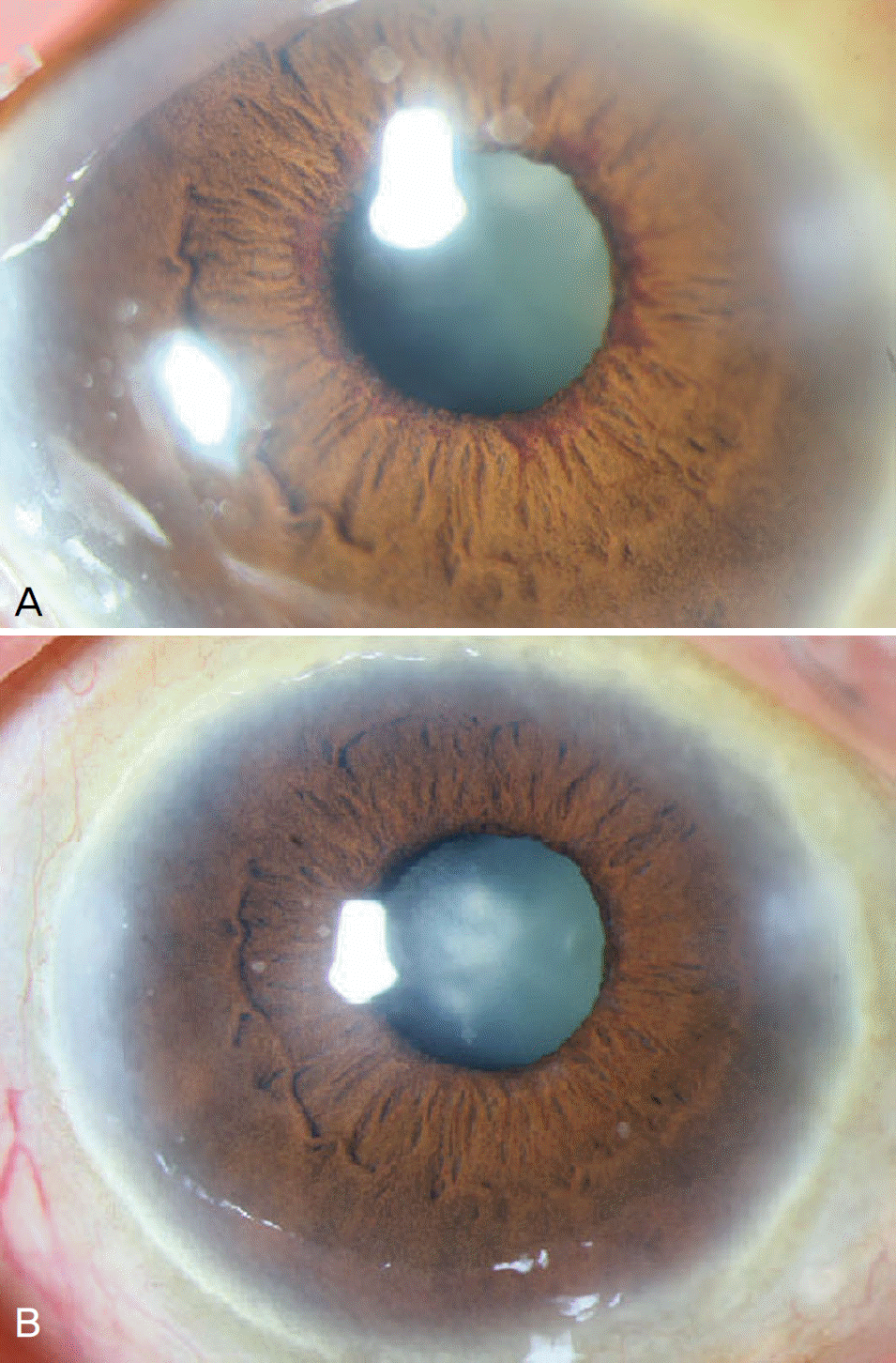
Table 1.
Characteristics of study population
| Characteristics | Data |
|---|---|
| Total patients (n*) | 15 |
| Male | 10 (67%) |
| Female | 5 (33%) |
| Age (y†) | 62.50±12.79 |
| Cause of Neovascular glaucoma (n) | |
| Diabetes Mellitus | 9 (60%) |
| Central retinal vein occlusion | 4 (27%) |
| Central retinal artery occlusion | 1 (7%) |
| Uveitis | 1 (7%) |
| Stage of Neovascular glaucoma (n) | |
| Open angle glaucoma‡ | 5(34%) |
| Closed angle glaucoma§ | 10(67%) |
Table 2.
Clinical evaluation of BCVA, IOP and neovascularization
| Patient | Angle | BC | VA* | IOP | P† (mm Hg) | NVI‡ (2M) | |
|---|---|---|---|---|---|---|---|
| Pre-injection | 2M†† | Pre-injection | 1M | 2M | |||
| 1 | Closed | LPП | LP | 46 | 14 | 15 | Regression |
| 2 | Open | NLP§ | NLP | 43 | 5 | 8 | Regression |
| 3 | Closed | NLP | NLP | 39 | 44 | 46 | Regression |
| 4 | Closed | NLP | NLP | 32 | 23 | 25 | Regression |
| 5 | Closed | NLP | NLP | 46 | 33 | 34 | Regression |
| 6 | Closed | NLP | NLP | 40 | 23 | 21 | Regression |
| 7 | Closed | NLP | NLP | 48 | 42 | 44 | Recurrence |
| 8 | Closed | NLP | NLP | 47 | 38 | 36 | Regression |
| 9 | Open | HM | HM | 38 | 34 | 32 | Regression |
| 10 | Closed | NLP | NLP | 35 | 37 | 35 | Regression |
| 11 | Open | HM# | FC**30cm | 28 | 17 | 15 | Regression |
| 12 | Closed | NLP | NLP | 52 | 42 | 43 | Regression |
| 13 | Closed | NLP | NLP | 50 | 30 | 28 | Recurrence |
| 14 | Open | 0.1 | 0.1 | 40 | 17 | 15 | Regression |
| 15 | Open | HM | 0.1 | 23 | 15 | 14 | Regression |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download