Abstract
Purpose
To introduce the clinical utility of multifocal visual evoked potential (mfVEP) and to assess the waveform from normal Korean subjects.
Methods
mfVEP with 4 channel recording was performed using the RETIscan® system (Roland Consult, Wiesbaden, Germany) for 25 eyes of 25 normal subjects. Amplitudes and implicit times were obtained from ring-shaped 6 areas and 4 sectors. To investigate the false-positive ratio of the examination, stimuli were given with one-half of the CRT monitor completely covered and the results were compared.
References
2. Odom JV, Bach M, Barber C, et al. Visual evoked potentials abdominal (2004). Doc Ophthalmol. 2004; 108:115–23.
3. Oh YD, Kwak HW, Kim SM. Evaluation of clinically applied VEP (visual evoked potential) in ophthalmological and abdominal diseases. J Korean Ophthalmol Soc. 1985; 26:1041–5.
4. Halliday AM, McDonald WI, Mushin J. Delayed visual evoked abdominal in optic neuritis. Lancet. 1972; 1:982–5.
5. Halliday AM, McDonald WI, Mushin J. Visual evoked response in diagnosis of multiple sclerosis. Br Med J. 1973; 4:661–4.

6. Halliday AM, Michael WF. Changes in pattern-evoked responses in man associated with the vertical and horizontal meridians of the visual field. J Physiol. 1970; 208:499–513.

7. Harding GF, Odom JV, Spileers W, et al. Standard for visual evoked potentials 1995. International Society for Clinical Electrophysiology of Vision. Vision Res. 1996; 36:3567–72.
8. Fortune B, Hood DC. Comparison of conventional and abdominal VEPs. Invest Ophthalmol Vis Sci. 2003; 44:1364–75.
9. Winn BJ, Shin E, Odel JG, et al. Interpreting the multifocal visual evoked potential: the effects of refractive errors, cataracts, and abdominal errors. Br J Ophthalmol. 2005; 89:340–4.
10. Klistorner AI, Graham SL. Objective perimetry in glaucoma. Ophthalmology. 2000; 107:2283–99.
11. Hood DC, Zhang X, Greenstein VE, et al. An interocular abdominal of the multifocal VEP: a possible technique for detecting local damage to the optic nerve. Invest Ophthalmol Vis Sci. 2000; 41:1580–7.
12. Fortune B, Zhang X, Hood DC, et al. Normative ranges and abdominal of the multifocal VEP. Doc Ophthalmol. 2004; 109:87–100.
13. Sutter EE. Imaging visual function with the multifocal m-se-quence technique. Vision Res. 2001; 41:1241–55.

14. Baseler HA, Sutter EE, Klein SA, Carney T. The topography of visual evoked response properties across the visual field. Electroencephlogr Clin Neurophysiol. 1994; 90:65–81.

15. Graham SL, Klistorner AI, Grigg JR, et al. Obejective VEP abdominal in glaucoma: asymmetry analysis to identity early deficits. J Glaucoma. 2000; 9:10–9.
16. Hood DC, Odel JG, Zhang X. Tracking the recovery of local abdominal nerve function after optic neuritis: a multifocal VEP study. Invest Ophthalmol Vis Sci. 2000; 41:4032–8.
17. Hood DC, Greenstein VC, Odel JG, et al. Visual field defects and multifocal visual evoked potentials. Arch Ophthalmol. 2002; 120:1672–81.

18. Hood DC. Assessing retinal function with the multifocal technique. Prog Retin Eye Res. 2000; 19:607–46.

19. Hood DC, Odel JG, Winn BJ. The multifocal visual evoked potential. J Neuroophthalmol. 2003; 23:279–89.

20. Fortune B, Hood DC, Johnson CA. Comparison of conventional and multifocal VEPs. Invest Ophthalmol Vis Sci. 2003; 44:1364–75.
21. Zhang X, Hood DC. A principle component analysis of abdominal pattern reversal VEP. J Vis. 2004; 4:32–43.
22. Hood DC, Zhang X, Hong JE, Chen CS. Quantifying the benefits of additional channels of multifocal VEP recording. Doc Ophthalmol. 2002; 104:303–20.
23. Daniel PM, Whitterridge W. The representation of the visual field on the cerebral cortex in monkeys. J Physiol. 1961; 159:203–21.

24. Horton JC, Hoyt WF. The representation of the visual field in abdominal striate cortex: revision of the classic Holmes map. Arch Ophthalmol. 1991; 109:816–24.
Figure 2.
The position of the four electrodes and the configuration of the four channel recording of mfVEP.
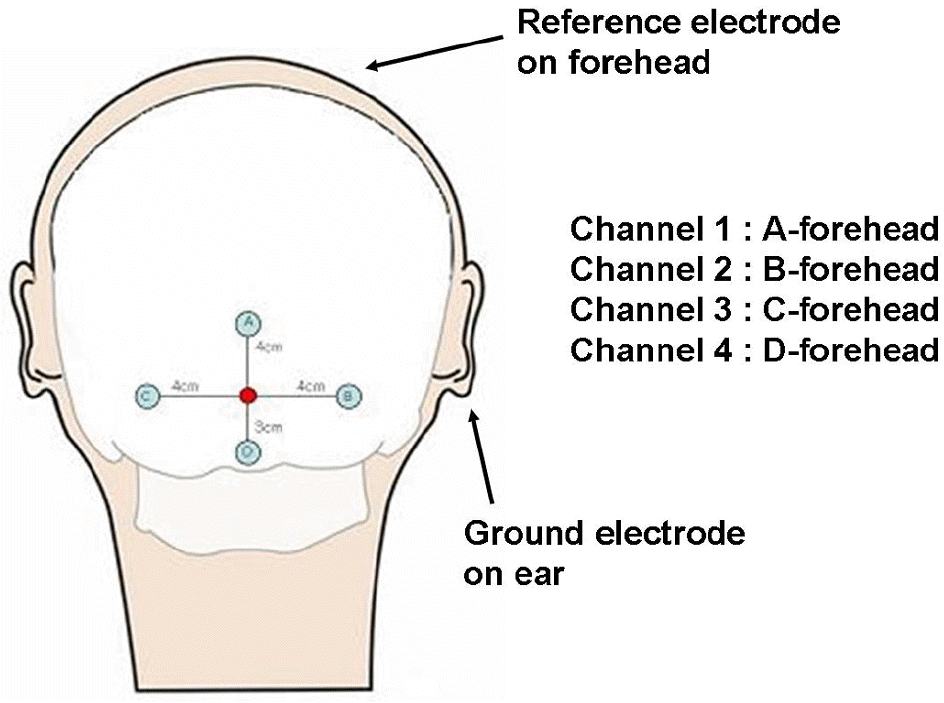
Figure 4.
To investigate the false-positive ratio of the examination, stimuli were given with covering one-half of the CRT monitor completely to stimulate the temporal retina of the right eye.
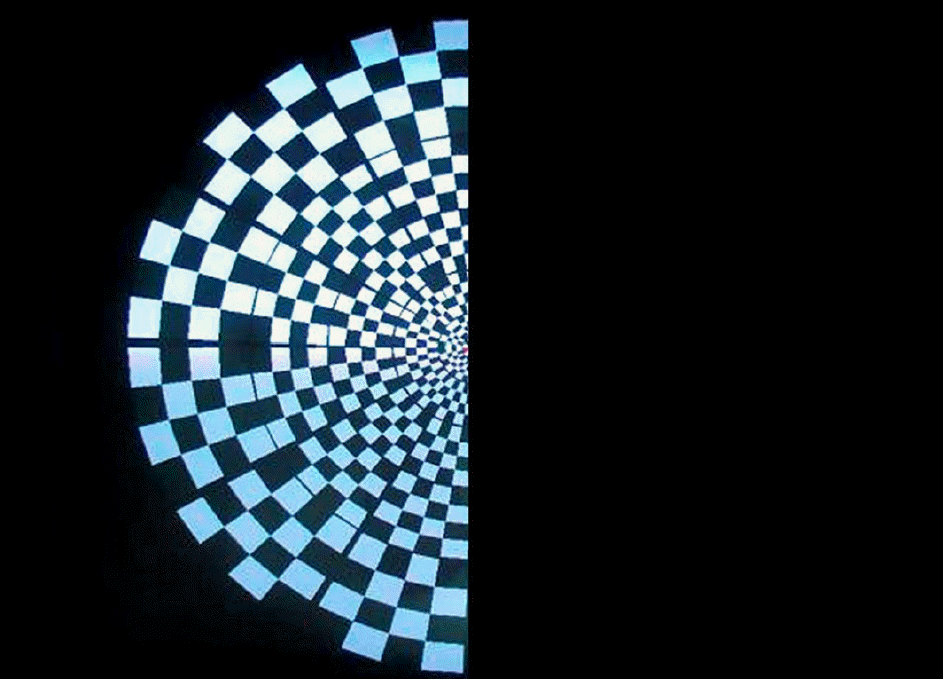
Figure 5.
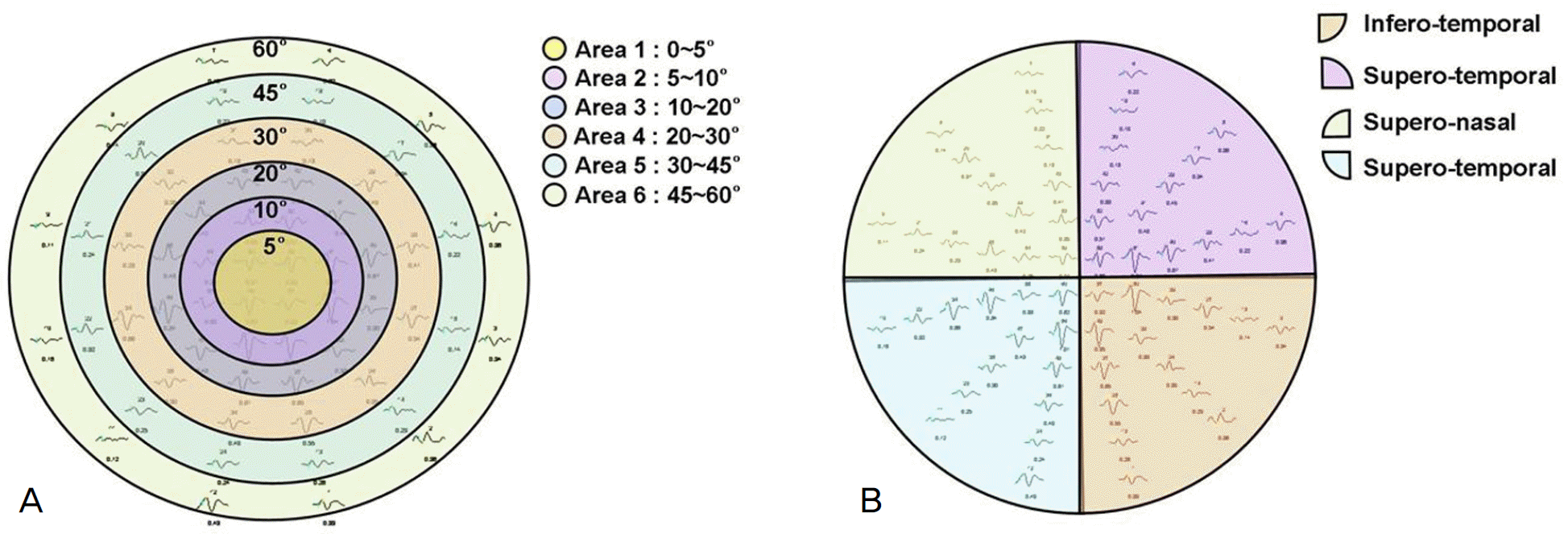
P1 (A) and N2 (B) amplitudes and P1 (C) and N2 (D) implicit times from mfVEP on 6 areas of visual field. P1 and N2 amplitudes on area 1 and 2 (*) were significantly higher than on the other areas (p<0.001, p<0.001). Implicit times of P1 and N2 showed no significant differences among areas (p=0.217, p=0.101).
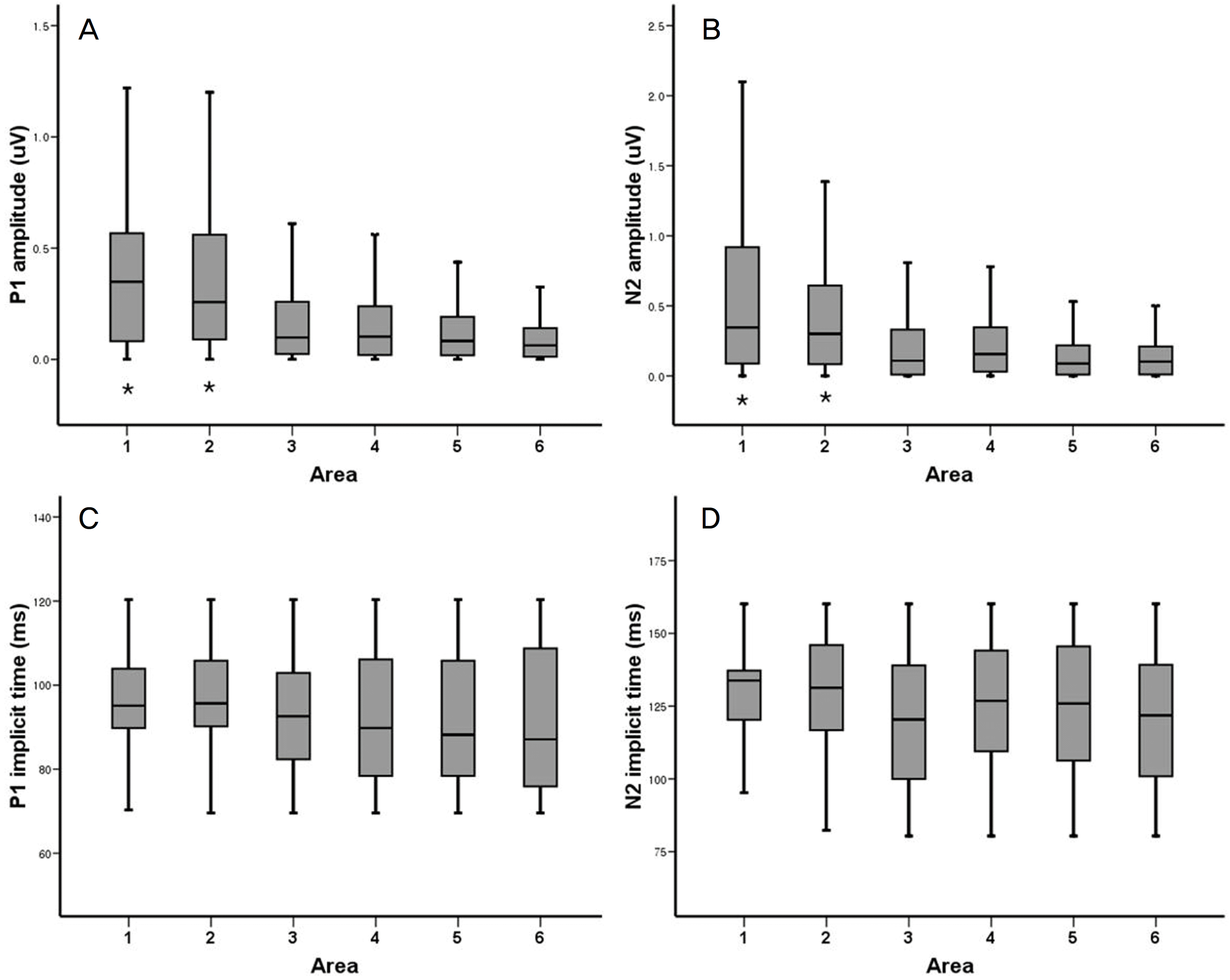
Figure 6.
P1 (A) and N2 (B) amplitudes and P1 (C) and N2 (D) implicit times from mfVEP on 4 sectors of visual field. P1 amplitudes on the inferior field (*) were significantly higher than on the other sectors (p=0.007). But, other parameters showed no significant differences among sectors.
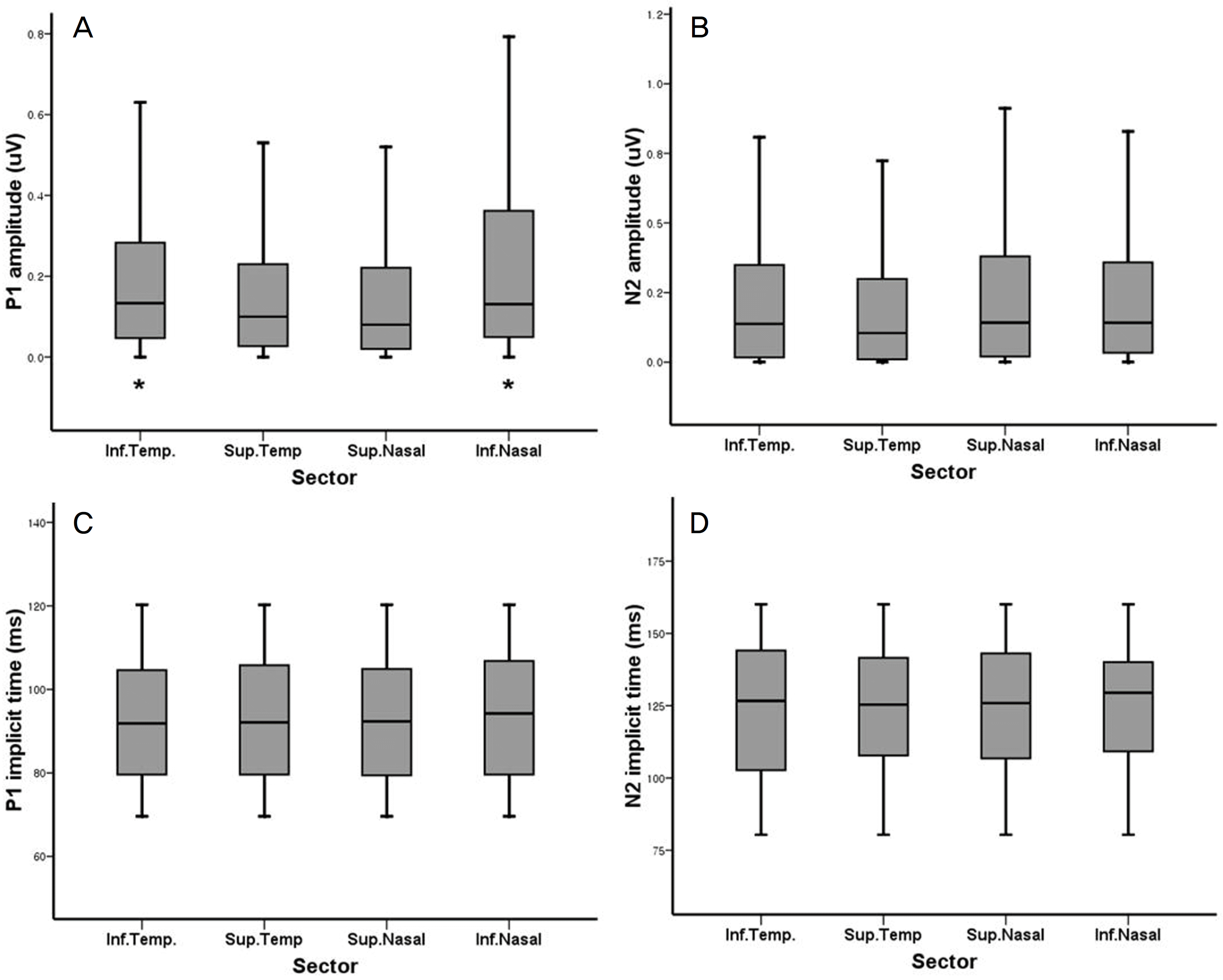
Figure 7.
The best VEP responses and 3-D plots of mfVEP without covering (A) and with covering on one-half of the CRT monitor (B). The amplitudes on no stimulation area were significantly smaller than on stimulation area (p<0.001). But, waves with large amplitudes were recorded on covered area in spite of no stimulation (five red hexagons).
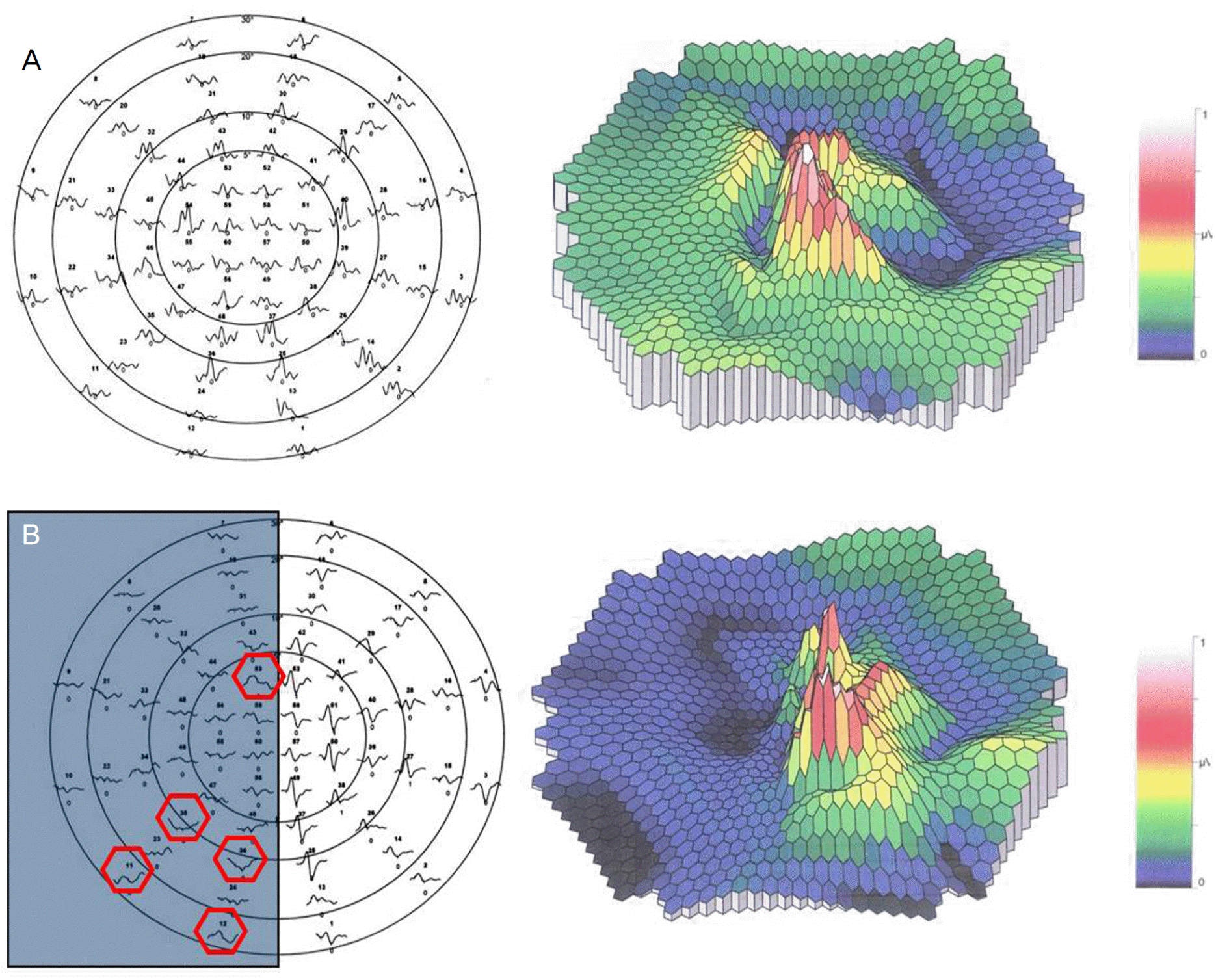
Figure 8.
Characteristics of results of multifocal VEP from normal subject. Signals with larger amplitudes were showed in central 15˚ field. Inferior field showed larger amplitudes compared to superior field. Signals out of 30˚ had relatively low amplitudes.
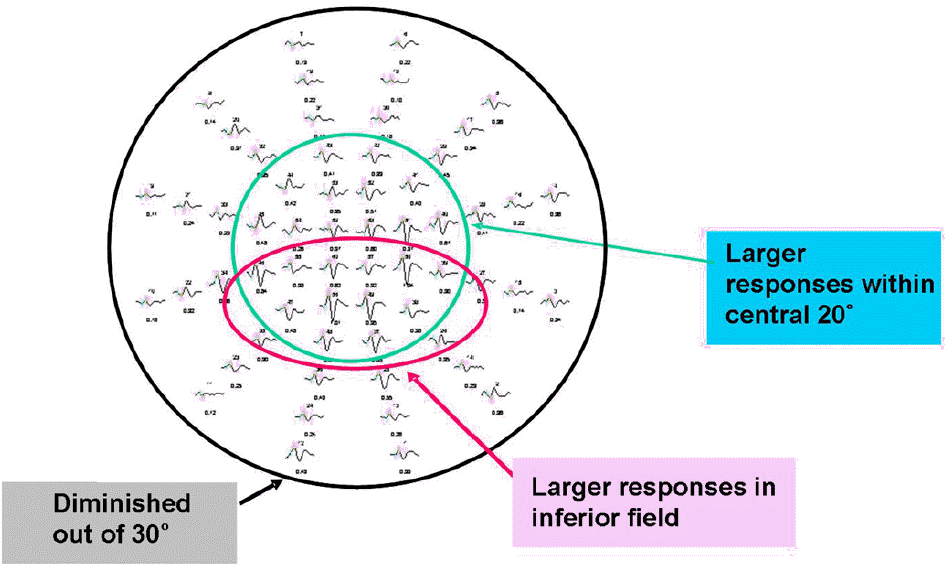
Table 1.
Mean amplitudes (μV) and implicit times (ms)of P1 and N2 of mfVEP on 6 areas
| Area 1 | Area 2 | Area 3 | Area 4 | Area 5 | Area 6 | p-value* | |
|---|---|---|---|---|---|---|---|
| P1 amplitude | 0.400±0.362 | 0.347±0.304 | 0.171±0.194 | 0.170±0.196 | 0.127±0.140 | 0.094±0.103 | <0.001 |
| N2 amplitude | 0.565±0.614 | 0.471±0.519 | 0.242±0.322 | 0.233±0.253 | 0.150±0.178 | 0.141±0.159 | <0.001 |
| P1 implicit time | 93.88±11.98 | 94.64±13.37 | 93.74±14.96 | 92.56±16.14 | 93.92±15.50 | 92.94±17.91 | 0.217 |
| N2 implicit time | 120.21±17.49 | 123.77±19.43 | 120.22±23.51 | 124.56±23.19 | 124.45±24.08 | 120.47±23.49 | 0.101 |
Table 2.
Mean amplitudes (μV) and implicit times (ms) of P1 and N2 of mfVEP on 4 sectors
| Inferotemporal | Superotemporal | Superonasal | Inferonasal | p-value* | |
|---|---|---|---|---|---|
| P1 amplitude | 0.200±0.215 | 0.169±0.193 | 0.160±0.207 | 0.226±0.241 | <0.001 |
| N2 amplitude | 0.267±0.388 | 0.212±0.273 | 0.279±0.273 | 0.271±0.262 | 0.057 |
| P1 implicit time | 92.84±15.44 | 93.19±16.08 | 92.82±15.46 | 94.32±15.40 | 0.554 |
| N2 implicit time | 123.31±24.51 | 123.59±21.87 | 123.38±23.27 | 125.48±21.90 | 0.556 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download