Abstract
Purpose
To evaluate retinal damage following internal limiting membrane (ILM) peeling in macular hole and diabetic macular edema (DME) surgeries.
Methods
Forty-five eyes with macular holes and thirty-five eyes with DME underwent pars plana vitrectomy with ILM peeling. The structures of the ILM were investigated using transmission electron microscopy, and the grades of retinal tissue damage were analyzed. We additionally observed the clinicopathologic association of retinal damage with the development of retinal hemorrhage during ILM peeling and that seen with indocyanine green (ICG) staining.
Results
In all specimens, cellular fragments were observed on the retinal side of the ILM in both macular hole and DME patients. The thickness of the ILM in DME significantly increased (3.13±1.12 μ m compared with that in patients with macular holes (2.41±0.77 μ m, p=0.002). The frequency of minute retinal bleeding during ILM peeling was higher in macular hole patients (46.7%) than in those with diabetic macular edema (22.9%m p=0.028). Twenty-two eyes of 45 macular hole patients (48.9%) and 16 eyes of 35 DME patients (45.7%) had relative retinal damage. Overall, ILM performed in eyes which had minute bleeding during the peeling had more retinal damage (62.1%) than did those without hemorrhage (39.2%, p=0.049). ICG staining did not appear to influence retinal damage (p=0.81).
References
1. Tognetto D, Grandin R, Sanguinetti G, et al. Internal limiting aberrations removal during macular hole surgery: results of a aberrations retrospective study. Ophthalmology. 2006; 113:1401–10.
2. Gandorfer A, Messmer EM, Ulbig MW, Kampik A. Resolution of diabetic macular edema after surgical removal of the posterior hyaloid and the inner limiting membrane. Retina. 2000; 20:126–33.

3. Matsunaga N, Ozeki H, Hirabayashi Y, et al. Histopathologic evaluation of the internal limiting membrane surgically excised from eyes with diabetic maculopathy. Retina. 2005; 25:311–6.

4. Boulton M, Foreman D, Williams G, McLeod D. VEGF localisation in diabetic retinopathy. Br J Ophthalmol. 1998; 82:561–8.

5. Barile GR, Pachydaki SI, Tari SR, et al. The RAGE axis in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005; 46:2916–24.

6. Uemoto R, Yamamoto S, Aoki T, et al. Macular configuration determined by optical coherence tomography after idiopathic macular hole surgery with or without internal limiting membrane peeling. Br J Ophthalmol. 2002; 86:1240–2.

7. Stefánsson E. Ocular oxygenation and the treatment of diabetic retinopathy. Surv Ophthalmol. 2006; 51:364–80.

8. Margherio RR, Margherio AR, Williams GA, et al. Effect of perifoveal tissue dissection in the management of acute idiopathic full-thickness macular holes. Arch Ophthalmol. 2000; 118:495–8.

9. Aboutable T. Is removal of internal limiting membrane always necessary during surgery for refractory diffuse diabetic macular edema without evident epimacular proliferation? Klin Monatsbl Augenheilkd. 2006; 223:681–6.
10. Uemoto R, Yamamoto S, Takeuchi S. Changes in retinal pigment epithelium after indocyanine green-assisted internal limiting lamina peeling during macular hole surgery. Am J Ophthalmol. 2005; 140:752–5.

11. Ando F, Yasui O, Hirose H, Ohba N. Optic nerve atrophy after vitrectomy with indocyanine green-assisted internal limiting membrane peeling in diffuse diabetic macular edema. Adverse effect of ICG-assisted ILM peeling. Graefes Arch Clin Exp Ophthalmol. 2004; 242:995–9.
12. Horio N, Horiguchi M. Effect on visual outcome after macular hole surgery when staining the internal limiting membrane with indocyanine green dye. Arch Ophthalmol. 2004; 122:992–6.
13. Yamashita T, Uemura A, Kita H, Sakamoto T. Analysis of the retinal nerve fiber layer after indocyanine green assisted aberrations for idiopathic macular holes. Ophthalmology. 2006; 113:280–4.
14. Gandorfer A, Haritoglou C, Gass CA, et al. Indocyanine aberrations peeling of the internal limiting membrane may cause aberrations damage. Am J Ophthalmol. 2001; 132:431–3.
15. Schumann RG, Schaumberger MM, Rohleder M, et al. aberrations of the Vitreomacular Interface in Full-Thickness Idiopathic Macular Holes: A Consecutive Analysis of 100 Cases. Am J aberrations. 2006; 141:1112–9.
16. Radetzky S, Walter P, Fauser S, et al. Visual outcome of patients with macular edema after pars plana vitrectomy and indocyanine green-assisted peeling of the internal limiting membrane. Graefes Arch Clin Exp Ophthalmol. 2004; 242:273–8.

17. Halfter W, Reckhaus W, Kröger S. Nondirected axonal growth on basal lamina from avian embryonic neural retina. J Neurosci. 1987; 7:3712–22.

18. Newman EA. Regional specialization of retinal glial cell aberrations. Nature. 1984; 309:155–7.
19. Winter M, Eberhardt W, Scholz C, Reichenbach A. Failure of potassium siphoning by Müller cells: a new hypothesis of perfluorocarbon liquid-induced retinopathy. Invest Ophthalmol Vis Sci. 2000; 41:256–61.
20. Wolf S, Schnurbusch U, Wiedemann P, et al. Peeling of the basal membrane in the human retina: ultrastructural effects. aberrationsogy. 2004; 111:238–43.
21. Mitamura Y, Ohtsuka K. Relationship of dissociated optic nerve fiber layer appearance to internal limiting membrane peeling. Ophthalmology. 2005; 112:1766–70.

Figure 1.
Transmission electron microscopic findings of the internal limiting membrane (ILM) removed during macular hole surgery. Only a few very tiny cellular fragments (arrow) are observed on the retinal side (R) of the ILM. The vitreous side (V) shows a very smooth surface. (×3,000)
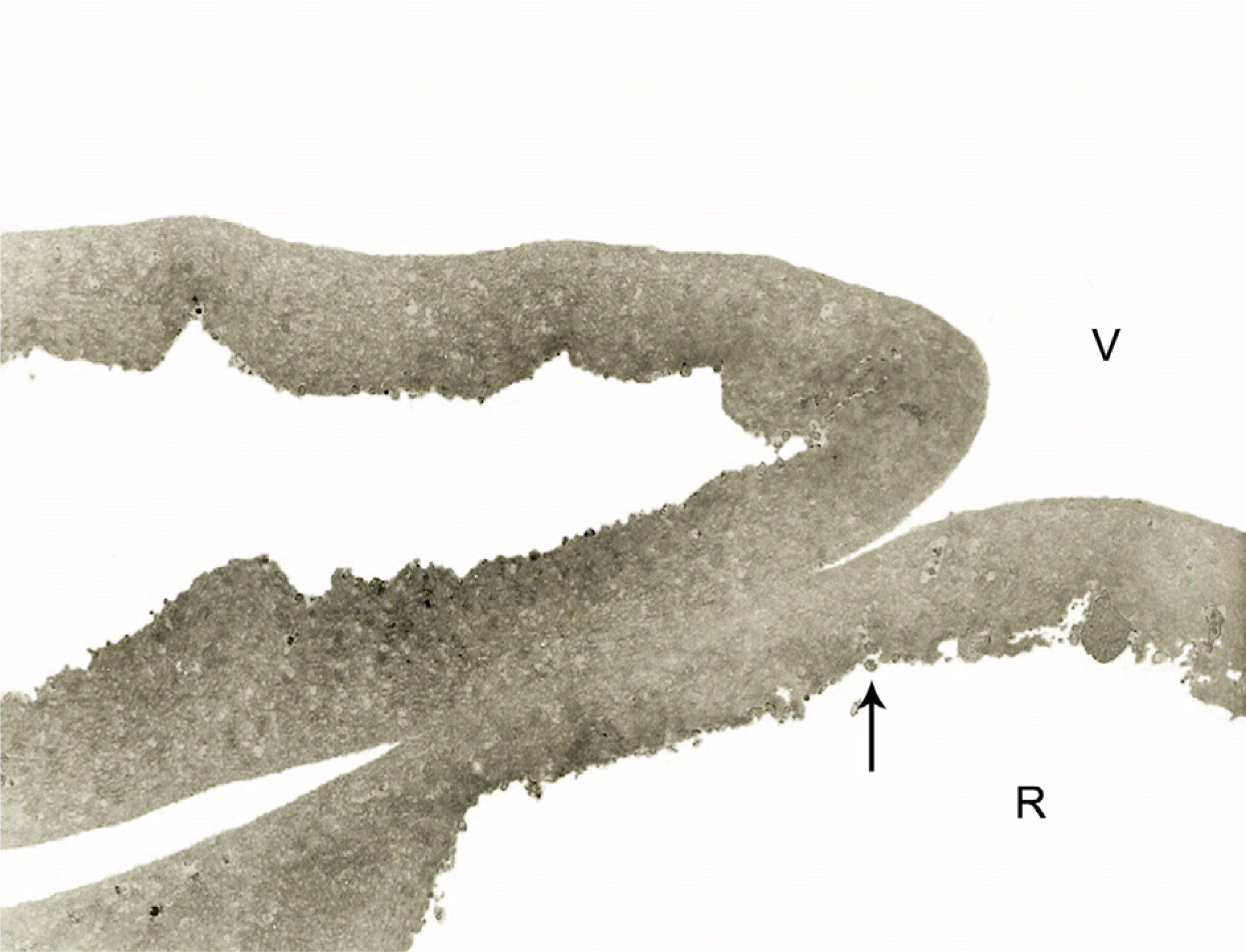
Figure. 2.
Many cellular debris of Müller cells (arrow) are adherent to the retinal surface (R) of the internal limiting membrane (asterisk) in diabetic macular edema. A red blood cell is visible. (arrowhead). Collagen and cellular elements cover the vitreous side (V) of the internal limiting membrane (×3,000).
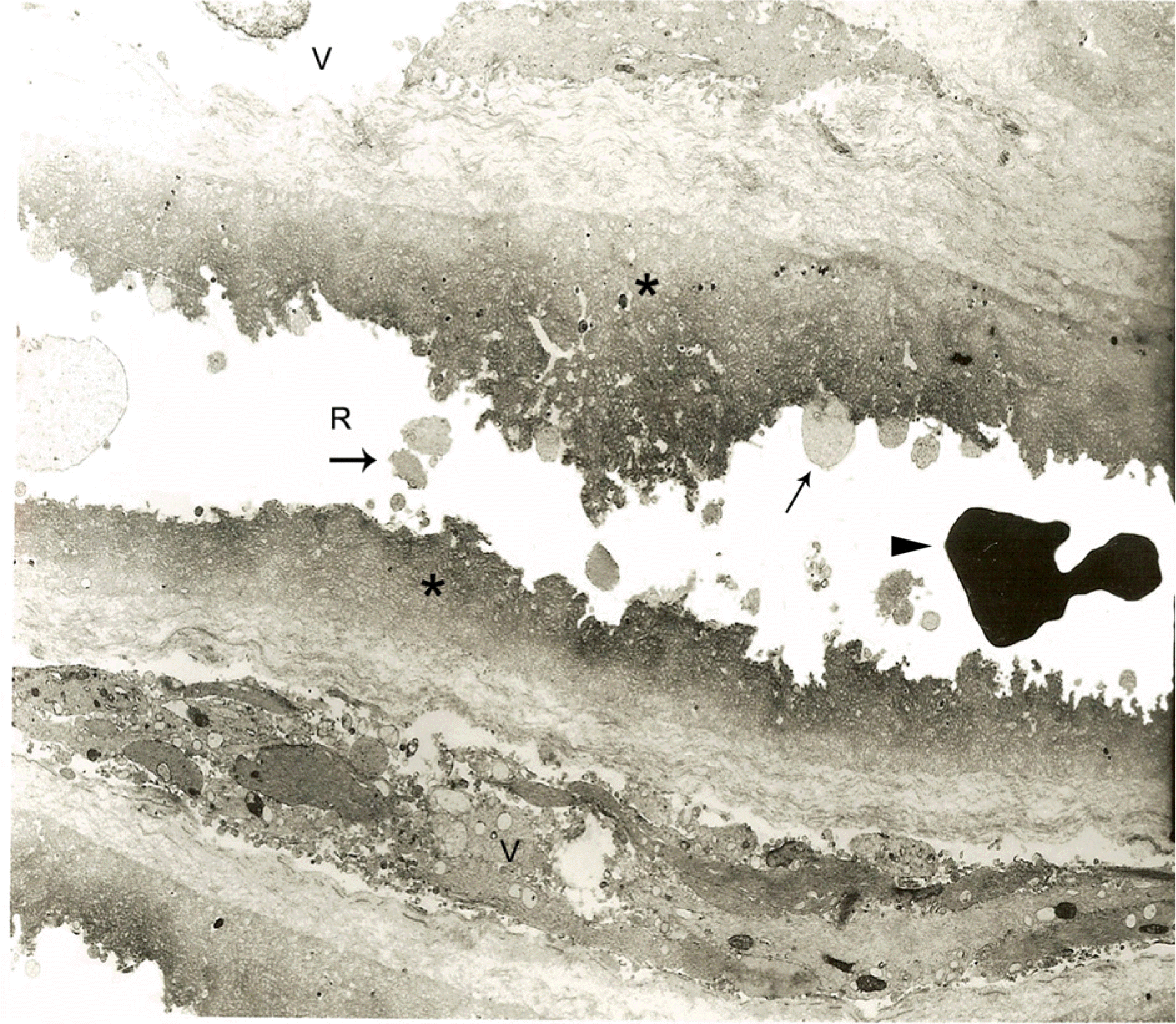
Figure 3.
Stretching forces split adjacent retinal structures (arrow) during the peeling of ILM in diabetic macular edema. The plasma membrane of Müller cells and other undetermined large cellular fragments (arrowhead) are adherent to the retinal surface of the ILM (×5,000).
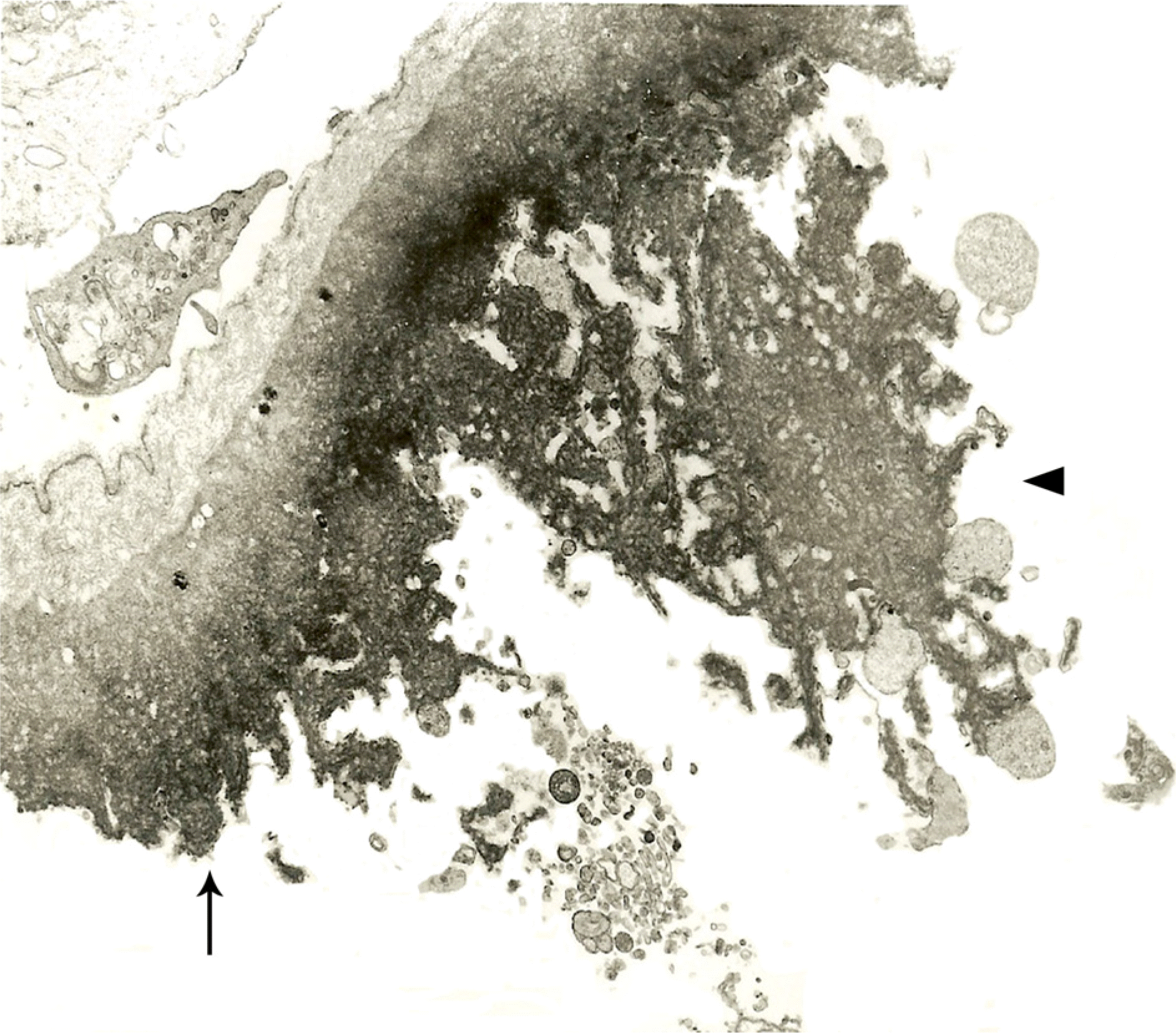
Figure 4.
Partial tear of the internal limiting membrane (arrow) is seen in diabetic macular edema (×6,000).
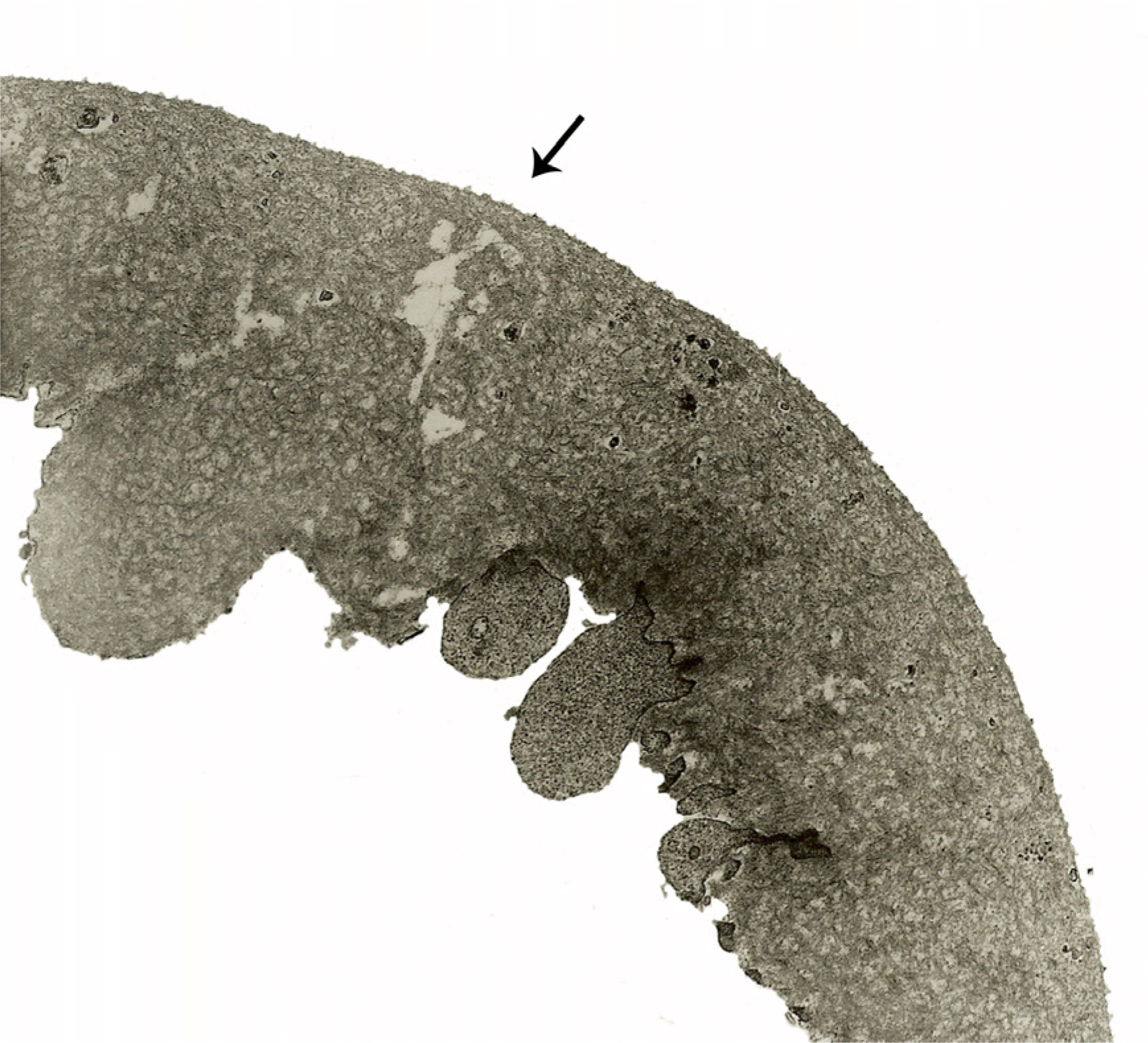
Table 1.
Demographic data
| Macular hole | |
|---|---|
| Age (yrs) | 63.3 (19∼85) |
| Sex (M/F) | 14/31 |
| Stage 2 | 8 |
| Stage 3 | 13 |
| Stage 4 | 24 |
| Diabetic macular edema | |
|---|---|
| Age (yrs) | 61.5 (50∼75) |
| Sex (M/F) | 19/16 |
| DM Duration (yrs) | 13.8 (±7.6) |
| Type 1/Type 2 | 0/35 |
| NPDR/PDR | 4/31 |
Table 2.
Mean ILM thickness, frequency of relative retinal damages and retinal hemorrhages in two groups
| | Macular hole (n=45) | Diabetic macular edema (n=35) | p-value |
|---|---|---|---|
| ILM thickness (μm) | 2.41±0.77 | 3.13±1.12 | 0.002* |
| Retinal damage | 22 (48.9) | 16 (45.7) | 0.78† |
| Retinal hemorrhage | 21 (46.7) | 8 (22.9) | 0.028† |
Table 3.
Association of retinal damage with development of retinal hemorrhage during ILM peeling
| |
Macular hole (n=45) |
Diabetic macular edema (n=35) |
Total (n=80) |
|||
|---|---|---|---|---|---|---|
| HM* (+) | HM (−) | HM (+) | HM (−) | HM (+) | HM (−) | |
| DMG† (+) | 12 (57.1) | 10 (41.7) | 6 (75.0) | 10 (37.0) | 18 (62.1) | 20 (39.2) |
| DMG (−) | 9 (42.9) | 14 (58.3) | 2 (25.0) | 17 (63.0) | 11 (37.9) | 31 (60.8) |
| Total | 21 (46.7) | 24 (53.3) | 8 (22.6) | 24 (77.4) | 29 (36.3) | 51 (63.7) |
| p-value | 0.30‡ | 0.11§ | 0.049‡ | |||




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download