Abstract
Purpose
We attempt to distinguish the patterns of macular edema due to branch retinal vein occlusion (BRVO) and to find correlations between the 24 hour short-term and three month long-term therapeutic effects of an intravitreal bevacizumab injection.
Methods
Forty-four eyes in 44 patients with macular edema due to BRVO underwent an intravitreal bevacizumab injection. Ophthalmoscopic examinations, fluorescein angiographic evaluations, and optical coherence tomography (OCT) examinations performed made before the injections, after 24 hours, and at one, two and three month follow-ups. OCT yielded three patterns of macular edema: diffuse macular edema, cystoid macular edema, and serous retinal detachment.
Results
Macular edema significantly improved 24 hours after the injections. The change in central macular thickness after 24 hours had a statistically significant correlation with the three month central macular thickness (Pearson correlation, r=0.757 p=0.011). Cystoid macular edema showed better improvement than the others after 24 hours, but no differences after three months.
Go to : 
References
1. Weinberg D, Dodwell DG, Fern SA. Anatomy of arteiovenous crossings in branch retinal vein occlusion. Am J Ophthalmol. 1990; 109:298–302.
2. The Branch Vein Occlusion Study Group. Argon laser photo-coagulation for macular edema in branch vein occlusion. Am J Ophthalmol. 1984; 98:271–82.
3. Ryan SJ. Retina. Dick SB, Jampol LM, Haller JA, editors. Macular edema. 3rd ed.St. Louis: Mosby Co;2001. 2:chap. 55–7.
4. Hee MR, Puliafito CA, Wong C, et al. Quantitative assessment of macular edema with optical coherence tomography. Arch Ophthalmol. 1995; 113:1019–29.

5. Konno S, Akiba J, Yoshida A. Retinal thickness measurements with optical coherence tomography and the scanning retinal thickness analyzer. Retina. 2001; 21:57–61.

6. Ghil HK, Cho SW, Kim SH. The effect of intravitreal triamcinolone acetonide for macular edema in branched retinal vein occlusion. J Korean Ophthalmol Soc. 2004; 45:2029–35.
7. Tolentino MJ Miller JW, Gragoudas ES, et al. Intravitreal injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996; 103:1820–8.
8. Noma H, Funatsu H, Yamasaki M, et al. Pathogenesis of macular edema with branched retinal vein obstruction and intraocular levels of vascular endothelial growth factor and interleukin-6. Am J Ophthalmol. 2005; 140:256–61.
9. Lee SW, Kim MS, Kim ES, et al. abdominal results of intravitreal bevacizumab injection for macular edema: retinal vein obstruction and diabetic retinopathy. J Korean Ophthalmol Soc. 2009; 50:211–8.
10. Iturralde D, Spaide RF, Meyerle CB, et al. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006; 26:279–84.
11. Kim JY, Kweon EY, Lee DW, et al. Results of intravitreal bevacizumab for macular edema with retinal vein occlusion and diabetic macular edema. J Korean Ophthalmol Soc. 2008; 49:1275–82.

12. Lee SW, Kim HK, Kim SY. Patterns of macular edema in patients with branched retinal vein occlusion on optical coherence tomography. J Korean Ophthalmol Soc. 2005; 46:969–75.
13. Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol. 1999; 127:688–93.

14. Finkelstein D. Ischemic macular edema. Recognition and favorable natural history in branch vein occlusion. Arch Ophthalmol. 1992; 110:1427–34.

15. Lerche RC, Schaudig U, Scholz F, et al. Structural changes of the retina in retinal vein occlusion-imaging and quantification with optical coherence tomography. Ophthalmic Surg Lasers. 2001; 32:272–80.

16. Aiello LP, Bursell SE, Clermont A, et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997; 46:1473–80.

17. Vinores SA, Youssri AO, Luna JD, et al. Upregulation of vascularendothelial growth factor in ischemic and nonischemic human and experimental retinal disease. Histol Histopathol. 1997; 12:99–109.
18. Pe'er J, Folberg R, Itin A, et al. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology. 1998; 105:412–6.
19. Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol. 1999; 237:130.

20. Aiello LP, Northrup JM, Keyt BA, et al. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol. 1995; 113:1538–44.

21. Wu L, Arevalo JF, Roca JA, et al. Pan-american collaborative retina study Group (PACORES). Comparison of two doses of intravitreal bevacizumab (Avastin) for treatment of macular edema secondary to branch retinal vein occlusion: results from the Pan-American Collaborative Retina Study Group at 6 months of follow-up. Retina. 2008; 28:212–9.
22. Rabena MD, Pieramici DJ, Castellarin AA, et al. Intravitrealbe-vacizumab (Avastin) in the treatment of macular edema secondaryto branch retinal vein occlusion. Retina. 2007; 27:419–25.
23. Badalà F. The treatment of branch retinal vein occlusion with bevacizumab. Curr Opin Ophthalmol. 2008; 19:234–8.

24. Shetty R, Pai SA, Vincent A, et al. Electrophysiological and structural assessment of the central retina following intravitreal injection of bevacizumab for treatment of macular edema. Doc Ophthalmol. 2008; 116:129–35.

25. Rich RM, Rosenfeld PJ, Puliafito CA, et al. abdominal safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina. 2006; 26:495–511.
26. Kim SJ, Park YM, Lee SU, et al. abdominal safety and efficacy of intravitreal bavacizumab injection. J Korean Ophthalmol Soc. 2009; 50:219–26.
27. Krohne TU, Eter N, Holz FG, Meyer CH. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am J Ophthalmol. 2008; 146:508–12.

28. Sung MS, Park TK, Ohn YH, et al. A correlation with retinal thickness using retinal thickness analyzer and the responses of multifocal electroretinogram in patients with diabetic macular edema. J Korean Ophthalmol Soc. 2006; 47:1401–9.
29. Kang SW, Park CY, Ham DI. The correlation between fluorescein angio and optical coherence tomographic features in clinically significant diabetic macular edema. Am J Ophthalmol. 2004; 137:313–22.
Go to : 
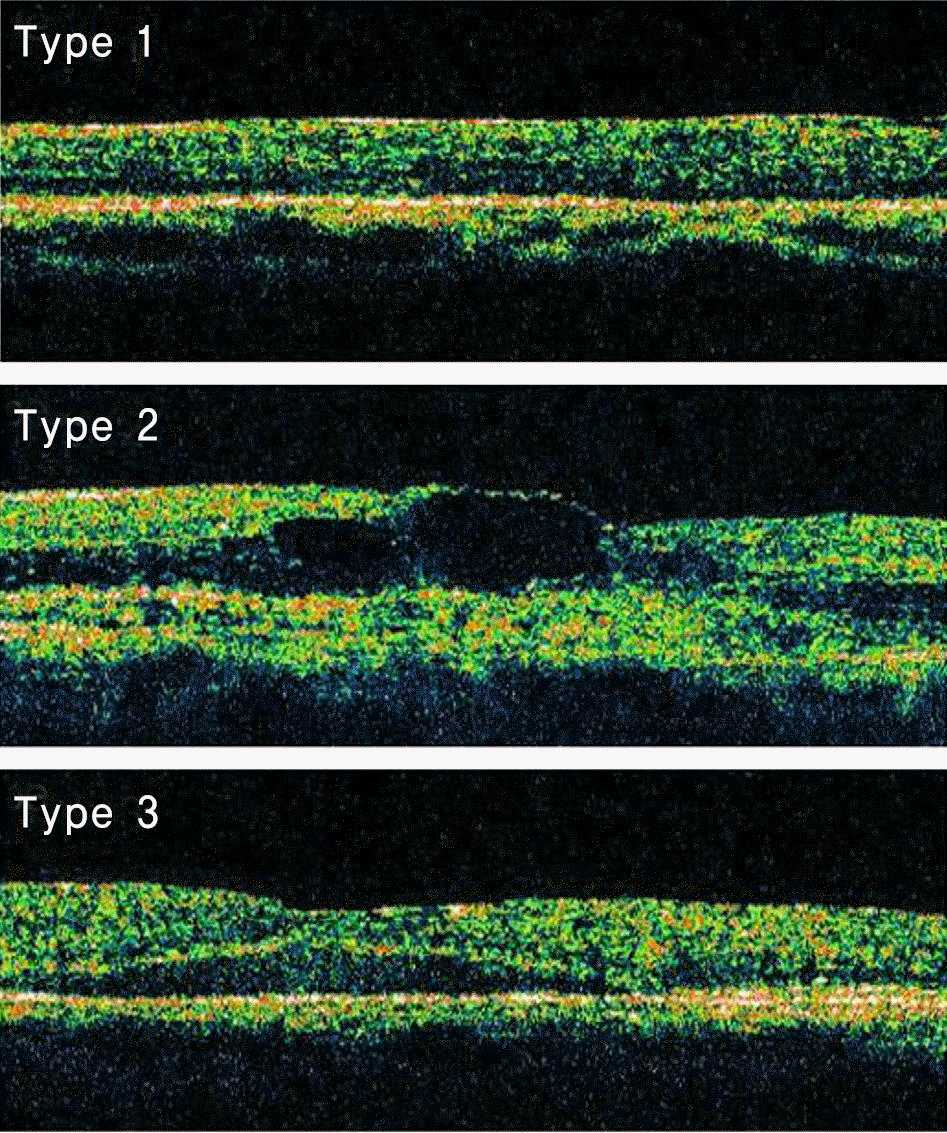 | Figure 1.Classification of macular edema in branch retinal vein occlusion patients: Type 1, diffuse macular edema. Type 2, cystoid macular edema. Type 3, serous retinal detachment. |
 | Figure 2.Correlation analysis of the visual acuity improvement at 24 hours after intravitreal bevacizumab injections with at 3 months (Pearson correlation, r=0.665; p=0.174). |
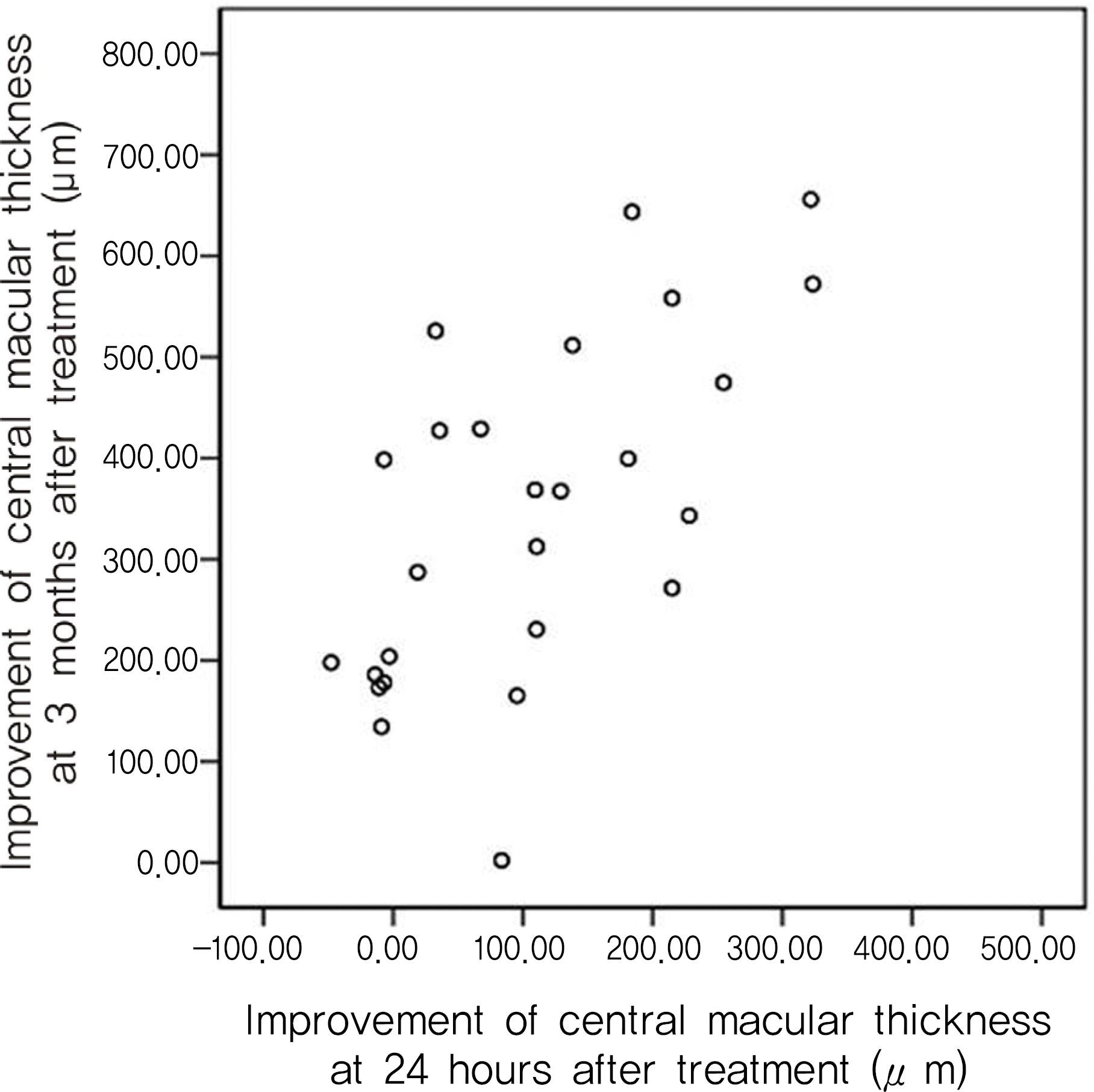 | Figure 3.Correlation analysis of the central macular thickness improvement at 24 hours after intravitreal bevacizumab injections with at 3 months. Strong correlation is observed (Pearson correlation, r=0.757; p=0.011). |
 | Figure 4.Inter-correlations between the improvement of 24 hours after intravitreal bevacizumab injection according to the OCT patterns of macular edema, in branch retinal vein occlusion patients. Cystoid macular edema showed better improvement than the others at 24 hoursb (p=student t-test; n=number of eyes); DME= diffuse macular edema (Type 1); CME=cystoid macular edema (Type 2); SRD=serous retinal detachment (Type 3). |
Table 1.
Baseline characteristics of branch retinal vein occlusion patients
| Variable | Total |
|---|---|
| Age (years, mean± SD*) | 62±10 |
| No. of eyes (%) | 44 (100%) |
| Sex (male: female) | 19: 25 |
| Mean follow up time (days) | 91 |
| IOP (mmHg, mean± SD) | 15.4±2.2 |
| Duration of symptom (days, mean± SD) | 22±18 |
| Preoperative BCVA† (LogMAR, mean± SD) | 0.76±0.43 |
| Preoperative CMT‡ (μm, mean± SD) | 578.0±65.0 |
Table 2.
Visual acuity and central macular thickness of eyes with macular edema from baseline through 3 months, in branch retinal vein occlusion patients treated with bevacizumab
| Variable | Baseline | 24 hours | 1month | 2months | 3months |
|---|---|---|---|---|---|
| BCVA† (LogMAR, mean± SD*) | 0.76±0.43 | 0.62±0.38 | 0.36±0.36 | 0.35±0.33 | 0.33±0.37 |
| p value§ | 0.079 | <0.001 | <0.001 | <0.001 | |
| CMT‡ (μm, mean± SD) | 578.0±65.0 | 460.5±77.7 | 311.1±80.3 | 289.3±84.2 | 268.5±108.1 |
| p value§ | <0.001 | <0.001 | <0.001 | <0.001 |
Table 3.
Baseline characteristics with different macular edema types in branch retinal vein occlusion patients
| Variable | DME‡ | CME§ | SRD∏ | p-value# |
|---|---|---|---|---|
| No. of eyes (%) | 13 (30%) | 19 (43%) | 12 (27%) | |
| Age (years, mean± SD*) | 59±8 | 60±9 | 68±15 | 0.312 |
| Mean follow up time (days) | 90 | 88 | 96 | 0.387 |
| IOP (mmHg, mean± SD) | 16.0±1.2 | 15.0±2.2 | 15.3±4.1 | 0.268 |
| Duration of symptom (days, mean± SD) | 34±16 | 19±19 | 13±5 | 0.032 |
| Preoperative BCVA† (LogMAR, mean± SD) | 0.85±0.63 | 0.75±0.33 | 0.67±0.29 | 0.209 |
| Preoperative CMT (μm, mean± SD) | 532.0±119.5 | 630.4±102.4 | 518.1±110.5 | 0.116 |
Table 4.
Visual acuity and central macular thickness of eyes with macular edema from baseline after 24 hours and 3 months, according to different macular edema types in branch retinal vein occlusion patients treated with bevacizumab
| Variable | Baseline | 24 hours | 3months |
|---|---|---|---|
| Diffuse macular edema | |||
| BCVA† (LogMAR, mean± SD*) | 0.85±0.63 | 0.60±0.53 | 0.37±0.44 |
| (p§=0.059) | (p§<0.001) | ||
| CMT‡ (μm, mean± SD) | 532.0±119.5 | 432.8±45.0 | 284.6±60.3 |
| (p§<0.001) | (p§<0.001) | ||
| Cystoid macular edema | |||
| BCVA (LogMAR, mean± SD) | 0.75±0.33 | 0.59±0.32 | 0.30±0.32 |
| (p§=0.057) | (p§<0.001) | ||
| CMT (μm, mean± SD) | 630.4±102.4 | 450.8±60.2 | 260.3±103.8 |
| (p§<0.001) | (p§<0.001) | ||
| Serous retinal detachment | |||
| BCVA (LogMAR, mean± SD) | 0.67±0.32 | 0.69±0.37 | 0.33±0.16 |
| (p§=0.160) | (p§<0.001) | ||
| CMT (μm, mean± SD) | 518.1±110.5 | 490.1±104.4 | 264.0±107.4 |
| (p§<0.001) | (p§<0.001) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download