Abstract
Purpose
To investigate the effect of high glucose (HG) on the production of reactive oxygen species (ROS) in retinal pigment epithelial (RPE) cells.
Methods
ARPE-19 cells were exposed to low glucose (5 mM) and high glucose (HG, 25 mM) for three days. Additionally, 50 μ M N-acetyl cysteine (NAC) and 100 μ M L-arginine were co-exposed. Productions of nitric oxide (NO), ROS, and superoxide were determined by Griess assay, DCFH-DA assay, and modified cytochrome c assay, respectively.
Go to : 
References
1. Wei YH. Oxidative stress and mitochondrial DNA mutations in human aging. Proc Soc Exp Biol Med. 1998; 217:53–63.

2. Sacca SC, Izzotti A, Rossi P, Traverso C. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res. 2007; 84:389–99.
3. Sacca SC, Pascotto A, Camicione P, et al. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005; 123:458–63.
4. Kawasaki A, Otori Y, Barnstable CJ. Müller cell protection of rat retinal ganglion cells from glutamate and nitric oxide aberrations. Invest Ophthalmol Vis Sci. 2000; 41:3444–50.
5. Morgan J, Caprioli J, Koseki Y. Nitric oxide mediates excitotoxic and anoxic damage in rat retinal ganglion cells cocultured with astroglia. Arch Ophthalmol. 1999; 117:1524–9.

6. Vorwerk CK, Hyman BT, Miller JW, et al. The role of neuronal and endothelial nitric oxide synthase in retinal excitotoxicity. Invest Ophthalmol Vis Sci. 1997; 38:2038–44.
7. Park GS, Kwon NS, Kim YM, Kim JC. The role of nitric oxide in ocular surface diseases. Korean J Ophthalmol. 2001; 15:59–66.

8. Becquet F, Courtois Y, Goureau O. Nitric oxide in the eye: Multifaceted roles and diverse outcomes. Surv Ophthalmol. 1997; 42:71–82.

9. Brodsky SV, Morrishow AM, Dharia N, et al. Glucose scavenging of nitric oxide. Am J Physiol Renal Physiol. 2001; 280:480–6.

10. El-Remessy AB, Abou-Mohamed G, Caldwell RW, Caldwell RB. High glucose-induced tyrosine nitration in endothelial cells: role of eNOS uncoupling and aldose reductase activation. Invest Ophthalmol Vis Sci. 2003; 44:3135–43.

11. Becker B. Diabetes and primary open-angle glaucoma. Am J Ophthalmol. 1971; 1:1–16.
12. Davies PD, Duncan G, Pynsent PB, et al. Aqueous humor glucose concentration in cataract patients and its effect on the lens. Exp Eye Res. 1984; 39:605–9.
13. Schraermeyer U, Heiman K. Current understanding on the role of retinal pigment epithelium and its pigmentation. Pigment Cell Res. 1999; 12:219–36.

14. Proctor PH, Reynolds ES. Free radicals and disease in man. Physiol Chem Phy Med NMR. 1984; 16:175–95.
15. Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996; 62:155–69.

16. Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.

17. Green LC, Wagner DA, Glogoski J, et al. Analysis of nitrate, nitrite and [15N]nitrate in biologic fluids. Anal Biochem. 1982; 126:131–8.
18. Beauchamp C, Fridovich L. Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal Biochem. 1971; 44:276–87.
19. Teufelhofer O, Weiss R-M, Parzefall W, et al. Promyelocytic HL60 cells express NADPH oxidase and are excellent targets in a rapid spectrophotometric microplate assay for extracellular aberrations. Toxicolol Sci. 2003; 76:376–93.
20. Joseph JA, Wang H. Quantifying cellular oxidative stress by di-chlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999; 27:612–16.
21. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991; 43:109–42.
22. Kim KO, Yoon TJ, Choi GJ. Induction of angiogenic cytokines in cultured RPE by oxidative stress. J Korean Ophthalmol Soc. 2004; 45:1742–9.
23. Chakravarthy U, Hayes RG, Stitt AW, et al. Constitutive nitric oxide synthase expression in retinal vascular endothelial cells is suppressed by high glucose and advanced glycation end products. Diabetes. 1998; 47:945–52.

24. Seigel GM, Chiu L, Paxhia A. Inhibition of neuroretinal cell death by insulinlike growth factor-1 and its analogs. Mol Vis. 2000; 6:157–63.
25. Barber AJ, Nakamura M, Wolpert EB, et al. Insulin rescues retinal neurons from apoptosis by a phosphatidyl 3-kinase/Akt-mediated mechanism that reduces the activation of Caspases-3. J Biol Chem. 2001; 276:32814–21.
Go to : 
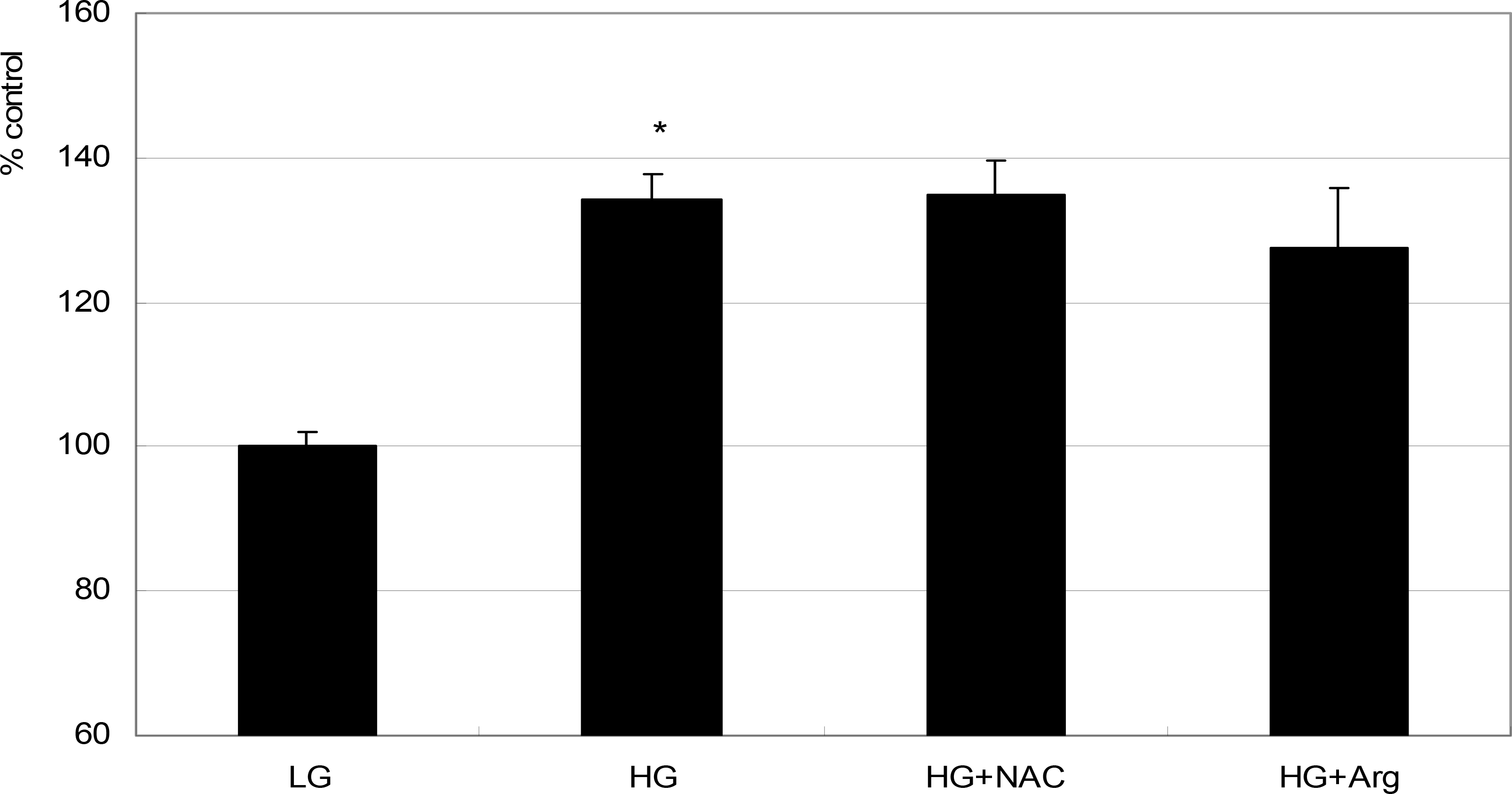 | Figure 1.Effect of high glucose on the survival of ARPE-19 cells. High glucose (HG, 25 mM) increased cellular survival significantly compared with low glucose (LG, 5 mM) (* p<0.05). Co-exposed 50 μM N-acetyl cysteine (NAC) or 100 μM L-arginine (L-Arg) did not affect the survival(p>0.05). |
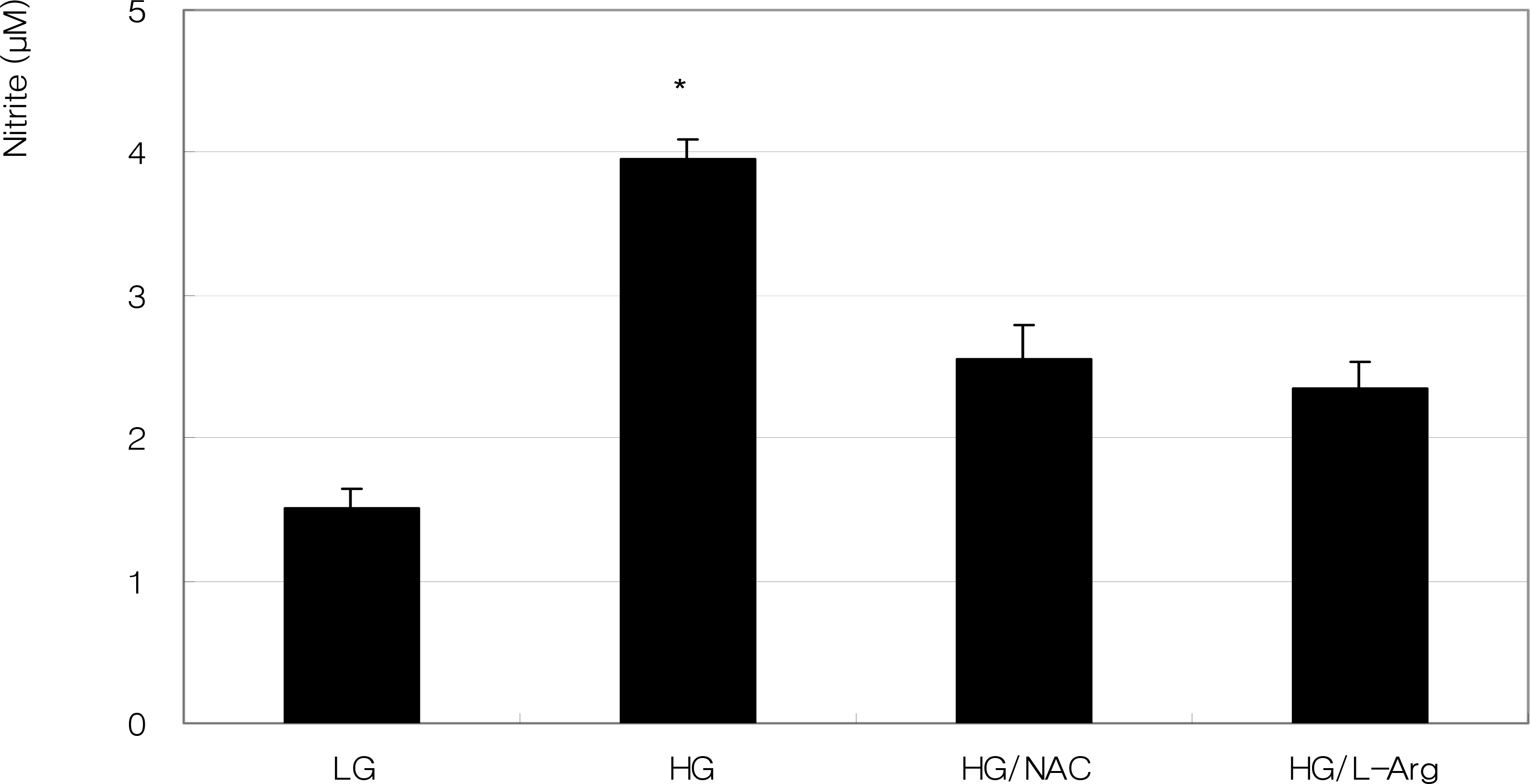 | Figure 2.Effect of high glucose on the production of nitric oxide in ARPE-19 cells. High glucose (HG, 25 mM) increased nitric oxide production significantly compared with low glucose (LG, 5 mM) (* p<0.05). Co-exposed 50 μM N-acetyl cysteine (NAC) or 100 μM L-arginine (L-Arg) did not affect the production of nitric oxide compared to HG alone (p>0.05). |
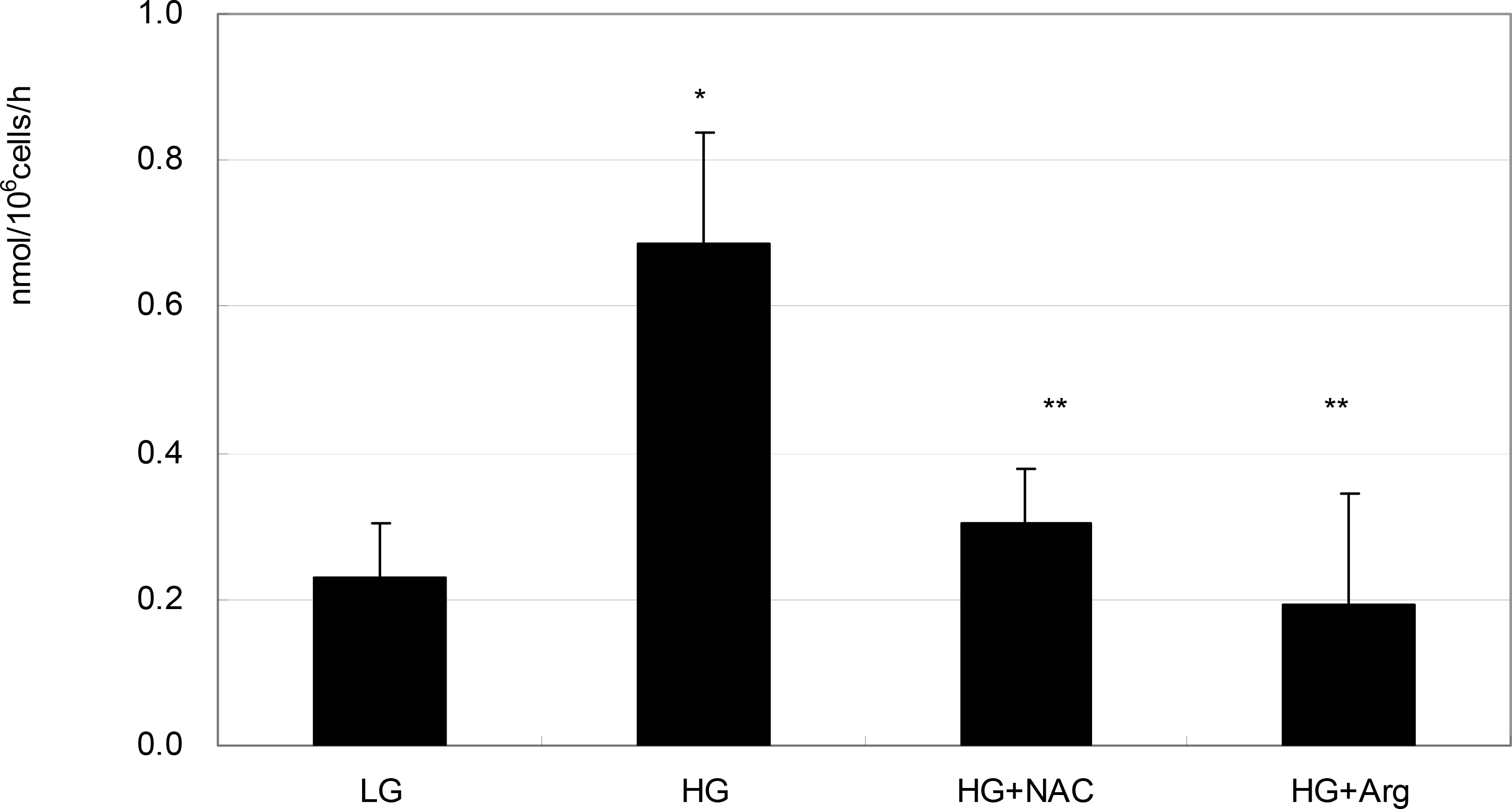 | Figure 3.Effect of high glucose on the production of superoxide in ARPE-19 cells. High glucose (HG, 25 mM) increased superoxide production significantly compared with low glucose (LG, 5 mM) (* p<0.05). HG-induced increased production of superoxide was abolished by co-exposed 50 μM N-acetyl cysteine (NAC) or 100 μM L-arginine (L-Arg), respectively (** p<0.05). |
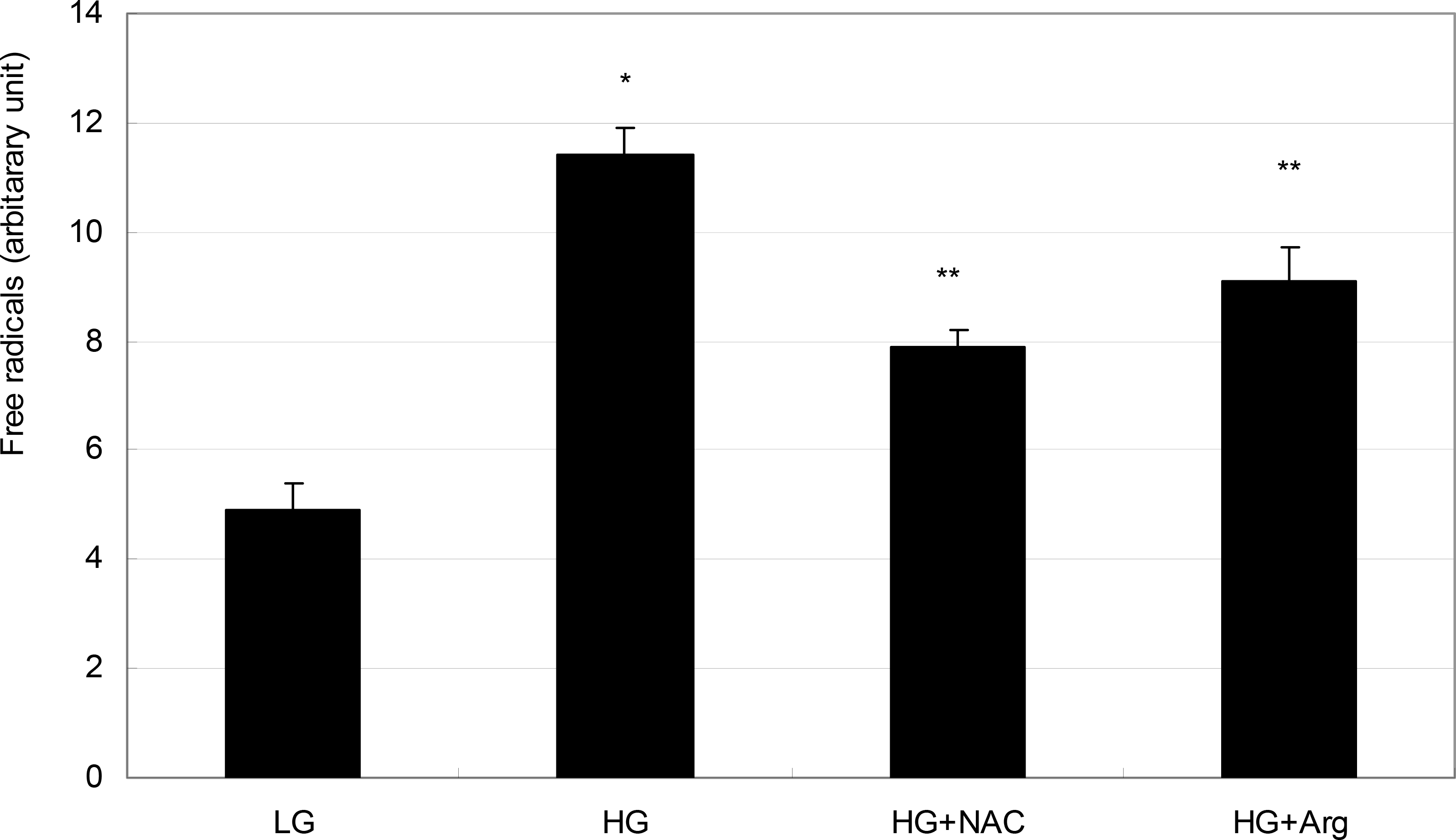 | Figure 4.Effect of high glucose on the production of reactive oxygen species (ROS) in ARPE-19 cells. High glucose (HG, 25 mM) increased ROS production significantly compared with low glucose (LG, 5 mM) (* p<0.05). HG-induced increased production of ROS was abolished by co-exposed 50 μM N-acetyl cysteine (NAC) or 100 μM L-arginine (L-Arg), respectively(** p<0.05). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download