Abstract
Purpose
To compare the effect of single intravitreal injection of triamcinolone acetonide and bevacizumab for the treatment of diabetic macular edema by optical coherence tomography (OCT) patterns.
Methods
We classified diabetic macular edema by three OCT patterns: Type 1, diffuse retinal thickening; Type 2, increased retinal thickness associated with the presence of intraretinal cystoid spaces (cystoid spaces with horizontal diameter >300 μ m); and Type 3, serous macular detachment. According to this classification, 84 eyes of 42 patients with bilateral diabetic macular edema by the same OCT pattern participated in this study. In each patient, one eye was treated with a single intravitreal injection of bevacizumab 1.25 mg/0.05 ml (IVBI group) and the other eye with a single intravitreal injection of 4 mg/0.1 ml triamcinolone (IVTI group). A comprehensive ophthalmic examination was performed at baseline and at one, three and six months after treatment. Main outcome measures included best corrected visual acuity (logMAR) and central macular thickness measured with OCT.
Results
In Type 1, central macular thickness (CMT) reduced significantly in the IVBI group at one month. In Type 2, mean BCVA and CMT improved significantly in the IVTA group at one and three months and in the IVBI group at one month following treatment. In Type 3, CMT reduced significantly in both groups at one, three and six months. However, BCVA did not significantly increase in either group at one, three or six months after treatment.
References
1. Moss SE, Klein R, Klein BE. The incidence of vision loss in a diabetic population. Ophthalmology. 1988; 95:1340–8.

2. McMeel JW, Trempe CL, Franks EB. Diabetic maculopathy. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977; 83:476–87.
3. Early Treatment Diabetic Retinopathy Study Report Number 1. Photocoagulation for diabetic macular edema. Arch Ophthalmol. 1985; 103:1796–805.
4. Martidis A, Duker JS, Greenberg PB, et al. Intravitreal aberrations for refractory diabetic macular edema. Ophthalmology. 2002; 109:920–7.
5. Massin P, Audren F, Haouchine B, et al. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology. 2004; 111:218–45.
6. Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007; 114:743–50.
7. Chun DW, Heier JS, Topping TM, et al. A pilot study of multiple intravitreal injections of ranibizumab in patients with center-involving clinically significant diabetic macular edema. aberrationsogy. 2006; 113:1706–12.

8. Funatsu H, Yamashita H, Ikeda T, et al. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to aberrations macular edema. Ophthalmology. 2003; 110:1690–6.
9. Funatsu H, Yamashita H, Sakata K, et al. Vitreous levels of aberrations endothelial growth factor and intercellular adhesion molecule 1 are related to diabetic macular edema. Ophthalmology. 2005; 112:806–16.
10. Hee MR, Izatt JA, Swanson EA, et al. Optical coherence aberrations of the human retina. Arch Ophthalmol. 1995; 113:325–32.
11. Konno S, Akiba J, Yoshida A. Retinal thickness measurements with optical coherence tomography and the scanning retinal aberrations analyzer. Retina. 2001; 21:57–61.
12. Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol. 1999; 127:688–93.

13. Kim YG, Yu SY, Kwak HW. The effect of intravitreal aberrations acetonide injection according to the diabetic macular edema type. J Korean Ophthalmol Soc. 2005; 46:84–9.
14. Yoon SC, Lee DY, Nam DH. The effect of intravitreal triamcinolone acetonide injection according to OCT patterns of diabetic macular edema. J Korean Ophthalmol Soc. 2008; 49:1611–8.
15. Jeong YC, Bae SH, Kim JW. Comparison of effects of IVTA and photocoagulation, depending on types of diabetic macular edema. J Korean Ophthalmol Soc. 2007; 48:655–64.
16. Shimura M, Nakazawa T, Yasuda K, et al. Comparative therapy evaluation of intravitreal bevacizumab and triamcinolone aberrations on persistent diffuse diabetic macular edema. Am J aberrations. 2008; 145:854–61.
17. Paccola L, Costa RA, Folgosa MS, et al. Intravitreal triamcinolone versus bevacizumab for treatment of refractory diabetic macular oedema (IBEME study). Br J Ophthalmol. 2008; 92:76–80.

18. Faghihi H, Roohipoor R, Mohammadi SF, et al. Intravitreal aberrations versus combined bevacizumab-triamcinolone versus aberrations laser photocoagulation in diabetic macular edema. Eur J aberrations. 2008; 18:941–8.
19. Koleva-Georgieva DN, Sivkova NP. Types of diabetic macular edema assessed by optical coherence tomography. Folia Med (Plovdiv). 2008; 50:30–8.
20. Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology. 1984; 91:1464–74.
21. Richter B, Kohner E. Medical interventions for diabetic aberrations. Wormald R, Smeeth L, Henshaw K, editors. Evidence-Based Ophthalmology. London: BMJ Books;2004. v. ?. chap. ?. p. 331–40.
22. Floman N, Zor U. Mechanism of steroid action in ocular aberrations: Inhibition of prostaglandin production. Invest aberrations Vis Sci. 1977; 16:69–73.
23. Edelman JL, Lutz D, Castro MR. Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Exp Eye Res. 2005; 80:249–58.

24. Jonas JB. Intravitreal triamcinolone acetonide for diabetic aberrations. Dev Ophthalmol. 2007; 39:96–110.
25. Adamis AP, Miller JW, Bernal MT, et al. Increased vascular aberrations growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994; 118:445–50.
26. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial aberrations factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331:1480–7.
27. Malecaze F, Clamens S, Simorre-Pinatel V, et al. Detection of aberrations endothelial growth factor messenger RNA and vascular aberrations growth factor-like activity in proliferative diabetic aberrations. Arch Ophthalmol. 1994; 112:1476–82.
28. Tolentino MJ, McLeod DS, Taomoto M, et al. Pathologic features of vascular endothelial growth factor-induced retinopathy in the nonhuman primate. Am J Ophthalmol. 2002; 133:373–85.

29. Tolentino MJ, Miller JW, Gragoudas ES, et al. Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in a nonhuman primate. Arch aberrations. 1996; 114:964–70.

30. Tolentino MJ, Miller JW, Gragoudas ES, et al. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996; 103:1820–8.

31. Funatsu H, Yamashita H, Noma H, et al. Increased levels of vascular endothelial growth factor and interleukin-6 in the aberrations humor of diabetics with macular edema. Am J Ophthalmol. 2002; 133:70–7.
32. Brooks HL Jr, Caballero S Jr, Newell CK, et al. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch Ophthalmol. 2004; 122:1801–7.
33. Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) aberrations of proliferative diabetic retinopathy complicated by aberrations hemorrhage. Retina. 2006; 26:275–8.
34. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal aberrations (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006; 113:363–72.
35. Costa RA, Jorge R, Calucci D, et al. Intravitreal bevacizumab for choroidal neovascularization caused by AMD: results of a phase I dose-escalation study. Invest Ophthalmol Vis Sci. 2006; 47:4569–78.
36. Kook D, Wolf A, Kreutzer T, et al. Long-term effect of intravitreal bevacizumab (avastin) in patients with chronic diffuse diabetic macular edema. Retina. 2008; 28:1053–60.

37. Fang X, Sakaguchi H, Gomi F, et al. Efficacy and safety of one intravitreal injection of bevacizumab in diabetic macular oedema. Acta Ophthalmol. 2008; 86:800–5.

38. Chang MW, Kim SW, Oh IK, et al. Intravitreal triamcinolone injection with or without bevacizumab for diabetic macular edema. J Korean Ophthamol Soc. 2008; 48:1269–74.

39. Kang SW, Park CY, Ham DI. The correlation between aberrations angio and optical coherence tomographic features in aberrations significant diabetic macular edema. Am J Ophthalmol. 2004; 137:313–22.
40. Brasil OF, Smith SD, Galor A, et al. Predictive factors for aberrations visual outcome after intravitreal triamcinolone acetonide injection for diabetic macular oedema: an optical coherence tomography study. Br J Ophthalmol. 2007; 91:761–5.
41. Kim BY, Smith SD, Kaiser PK. Optical coherence tomography patterns of diabetic macular edema. Am J Ophthalmol. 2006; 142:405–12.
42. Kumar A, Sinha S. Intravitreal bevacizumab (Avastin) treatment of diffuse diabetic macular edema in an Indian population. Indian J Ophthalmol. 2007; 55:451–5.

43. Kaiser PKk, Riemann CD, Sears JE, Lewis H. Macular traction detachment and diabetic macular edema associated with posterior hyaloidal traction. Am J Ophthalmol. 2001; 131:44–9.

44. Yamaguchi Y, Otani T, Kishi S. Resolution of diabetic cystoid macular edema associated with spontaneous vitreofoveal aberrations. Am J Ophthalmol. 2003; 135:116–8.
45. Yanoff M, Fine BS, Brucker AY, et al. Pathology of human cystoid macular edema. Surv Ophthalmol. 1984; 28:S505–11.

46. Shahar J, Avery RL, Heilweil G, et al. Electrophysiologic and aberrations penetration studies following intravitreal injection of aberrations (Avastin). Retina. 2006; 26:262–9.
47. Funatsu H, Yamashita H, Noma H, et al. Increased levels of aberrations endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002; 133:70–7.
48. Antcliff RJ, Marshall J. The pathogenesis of edema in diabetic aberrations. Semin Ophthalmol. 1999; 14:223–32.
49. Sutter FK, Simpson JM, Gillies MC. Intravitreal triamcinolone for diabetic macular edema that persists after laser treatment: three-month efficacy and safety results of a prospective, aberrations, double-masked, placebo-controlled clinical trial. aberrations. 2004; 111:2044–9.
50. Bonini-Filho MA, Jorge R, Barbosa JC, et al. Intravitreal injection versus sub-Tenon's infusion of triamcinolone acetonide for refractory diabetic macular edema: a randomized clinical trial. Invest Ophthalmol Vis Sci. 2005; 46:3845–9.

51. Haritoglou C, Kook D, Neubauer A, et al. Intravitreal aberrations (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006; 26:999–1005.
52. Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal aberrations (Avastin) in the treatment of proliferative diabetic aberrations. Ophthalmology. 2006; 113:1695–705.
53. Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of aberrations bevacizumab (Avastin). Ophthalmology. 2007; 114:855–9.
Figure 1.
The classification of diabetic macular edema on optical coherent tomographic images. (A) Diffuse thickening of the retina with homogeneous optical reflectivity on the whole layer (Type 1). (B) Macular edema with cystoid lesion that shows increased lower refrectivity in the retinal layer (Type 2). (C) Subretinal fluid accumulation with foveolar detachment without vitreoretinal traction (Type 3).

Figure 2.
Mean visual acuity change on both groups in the type 1 DME after intravitreal injection DME=Diabetic macular edema; IVTI=Intravitreal triamcinolone acetonide injection group; IVBI=Intravitreal bevacizumab injection group.
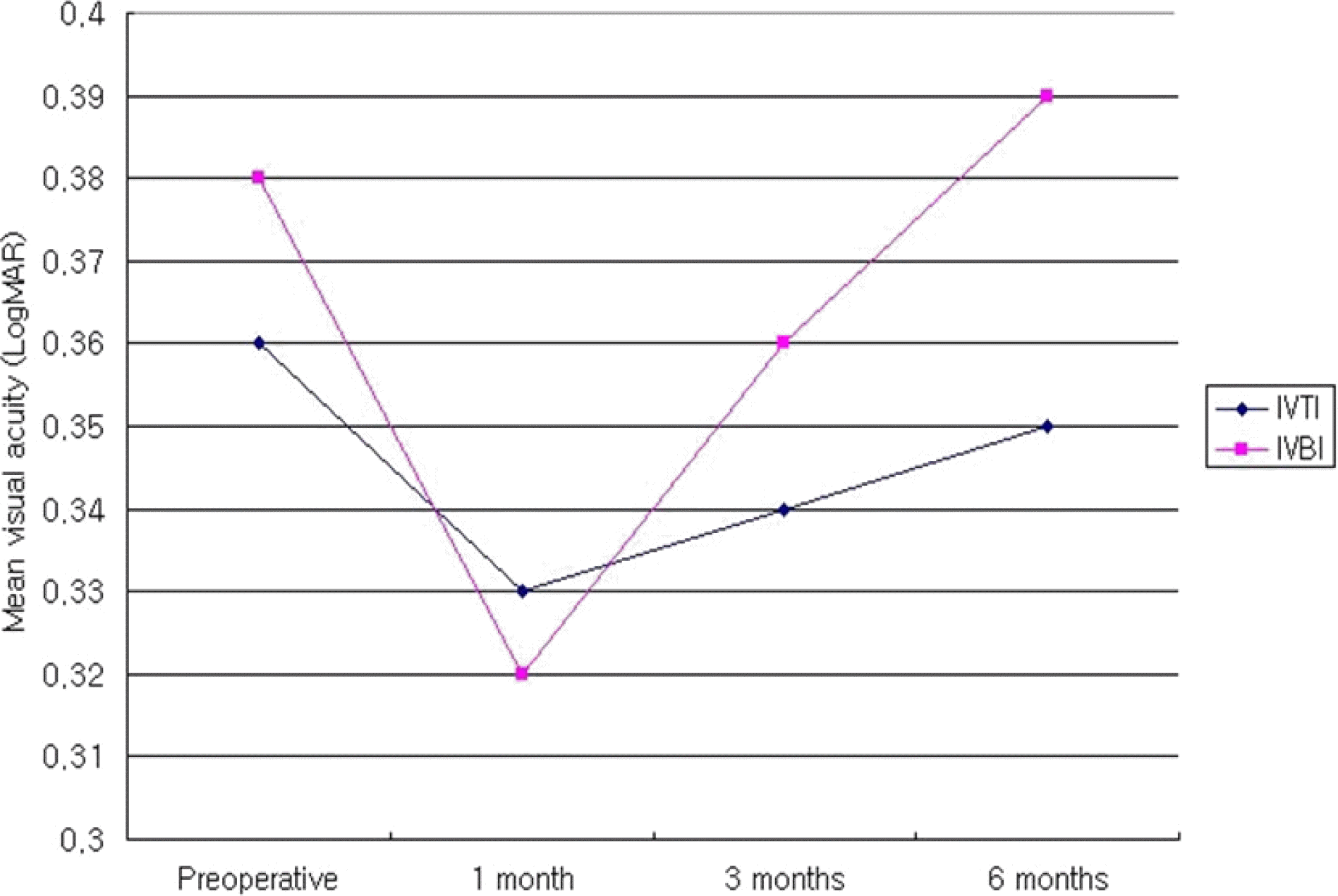
Figure 3.
Mean visual acuitv change on both groups in the type 2 DME after intravitreal injection DME=Diabetic macular edema; IVTI=Intravitreal triamcinolone acetonide injection group; IVBI=Intravitreal bevacizumab injection group.
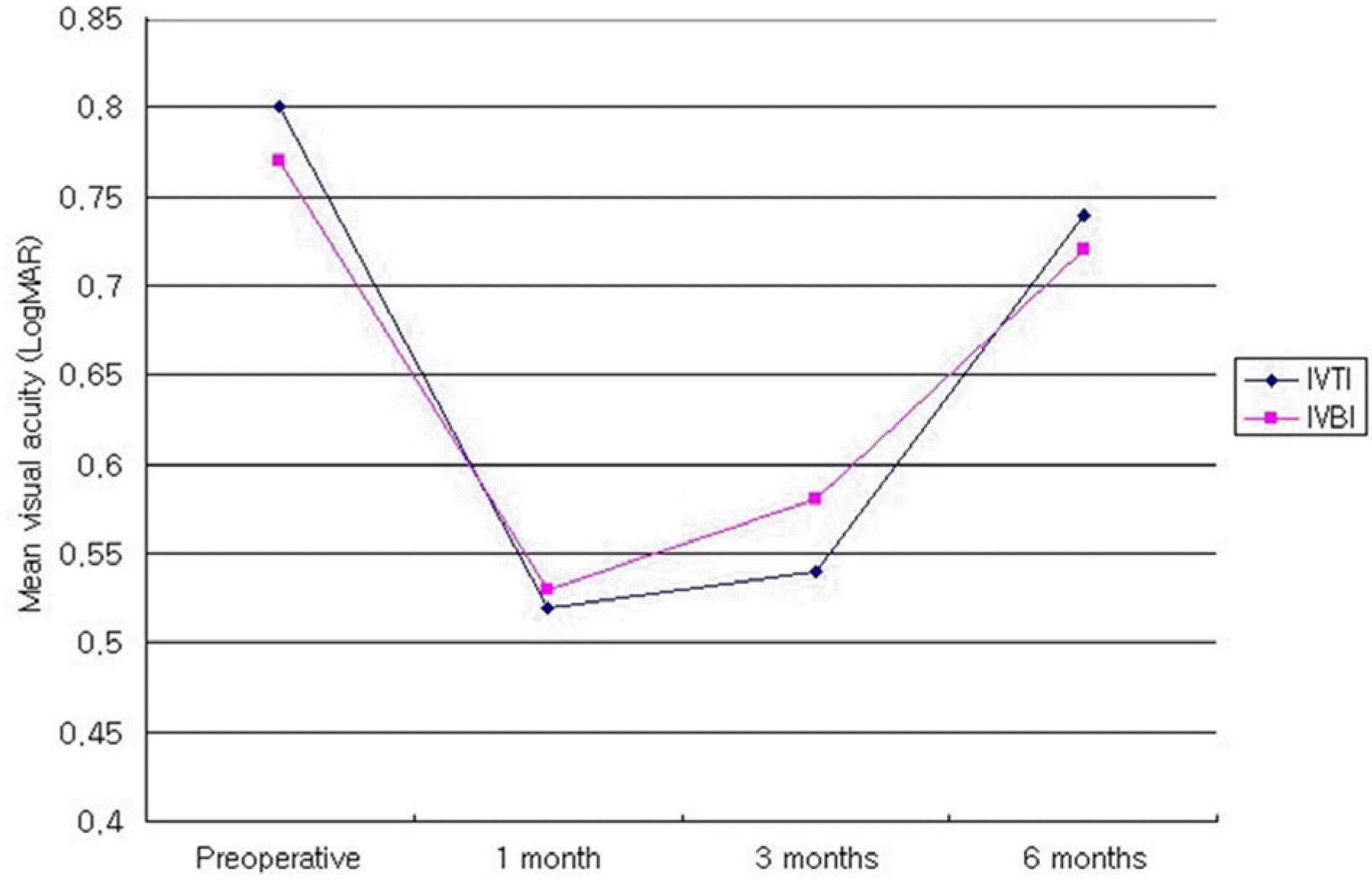
Figure 4.
Mean visual acuitv change on both groups in the type 3 DME after intravitreal injection DME=Diabetic macular edema; IVTI=Intravitreal triamcinolone acetonide injection group; IVBI=Intravitreal bevacizumab injection group.
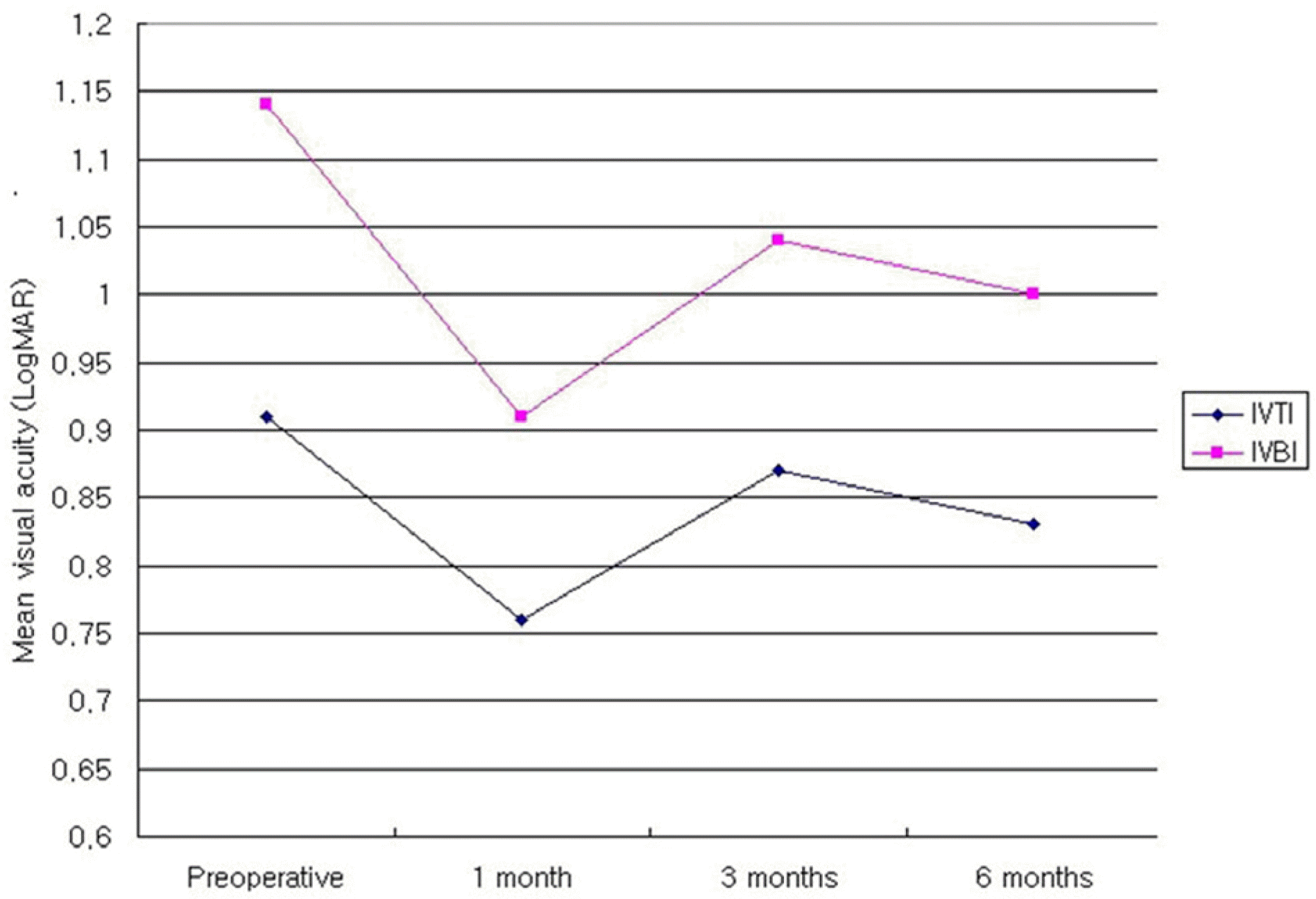
Table 1.
Clinical characteristics of patients
| Characteristic |
Type 1 |
Type 2 |
Type 3 |
|||
|---|---|---|---|---|---|---|
| IVTI† | IVBI‡ | IVTI† | IVBI‡ | IVTI† | IVBI‡ | |
| Age(years) | | | | | | |
| Mean ± SD | 60.0±14.4 | 57.7±13.8 | 61.2±9.7 | |||
| Range | 33 to 77 | 28 to 76 | 34 to 71 | |||
| Number of patients | 11 | 12 | 8 | |||
| Men | 6 | 5 | 3 | |||
| Women | 5 | 7 | 5 | |||
| Number of eyes | 22 | 24 | 16 | |||
| DM duration (years) | 13.4±9.6 | 12.6±2.8 | 10.8±6.3 | |||
| History of PRP(eyes) | 8 | 14 | 8 | |||
| DR status | | | | | | |
| Severe NPDR: number (eyes) | 8 (16) | 6 (12) | 4 (8) | |||
| PDR: number (eyes) | 3 (6) | 6 (12) | 4 (8) | |||
| Cataract Operation history | | | | | | |
| Phakic (eyes) | 8 | 8 | 10 | 10 | 7 | 7 |
| Pseudophakic (eyes) | 3 | 3 | 2 | 2 | 1 | 1 |
Table 2.
Best corrected visual acuity preoperative and postoperative 1, 3 and 6 months
| Types of DME | Intravitreal injection | Pre-operative BCVA (LogMAR) | Postoperative (1 Month) | p-value | Postoperative (3 Month) | p-value | Postoperative (6 Month) | p-value |
|---|---|---|---|---|---|---|---|---|
| Type 1 | IVTI†1 | 0.36±0.19 | 0.33±0.18 | 0.590 | 0.34±0.22 | 0.798 | 0.35±0.31 | 0.502 |
| | IVBI‡ | 0.38±0.27 | 0.32±0.23 | 0.303 | 0.36±0.31 | 0.502 | 0.39±0.23 | 0.673 |
| Type 2 | IVTI† | 0.80±0.53 | 0.52±0.34 | 0.007 | 0.54±0.26 | 0.041 | 0.74±0.41 | 0.646 |
| | IVBI‡ | 0.77±0.53 | 0.53±0.43 | 0.005 | 0.58±0.38 | 0.332 | 0.72±0.49 | 0.859 |
| Type 3 | IVTI† | 0.91±0.33 | 0.76±0.22 | 0.172 | 0.87±0.34 | 0.518 | 0.83±0.30 | 0.144 |
| | IVBI‡ | 1.14±0.36 | 0.91±0.30 | 0.006 | 1.04±0.27 | 0.357 | 1.00±0.27 | 0.141 |
Table 3.
Mean foveal thickness preoperative and postoperative 1, 3 and 6 months
| Types of DME | Intravitreal injection | Pre-operative (μm) | Postoperative (1 Month) | p-value | Postoperative (3 Month) | p-value | Postoperative (6 Month) | p-value |
|---|---|---|---|---|---|---|---|---|
| Type 1 | IVTI† | 241±35 | 208±27 | 0.091 | 218±24 | 0.450 | 240±39 | 0.374 |
| | IVBI‡ | 248±39 | 212±50 | 0.047 | 236±29 | 0.150 | 261±39 | 0.625 |
| Type 2 | IVTI† | 447±81 | 243±76 | 0.002 | 361±67 | 0.003 | 421±78 | 0.221 |
| | IVBI‡ | 473±82 | 323±72 | 0.009 | 412±56 | 0.285 | 431±61 | 0.131 |
| Type 3 | IVTI† | 594±139 | 284±80 | 0.028 | 421±79 | 0.028 | 519±135 | 0.018 |
| | IVBI‡ | 555±96 | 276±42 | 0.012 | 469±96 | 0.025 | 483±67 | 0.028 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download