Abstract
Purpose
To compare intraocular pressure (IOP) changes after intravitreal triamcinolone acetonide versus bevacizumab injection.
Methods
IOP was measured in 40 patients who received an intravitreal injection of 0.1 ml triamcinolone and 40 patients who received 2 consecutive intravitreal injections of bevacizumab (0.05 ml and 0.1 ml). Patients were divided into three groups: 0.1 ml triamcinolone group (0.1 IVTA group), 0.05 ml bevacizumab group (0.05 IVB group), and 0.1 ml bevacizumab group (0.1 IVB group). IOPs were compared within groups at different time points (before, 1 day after, and 30 days after the injection) and between groups at the same time points.
Results
In the 0.05 IVB and 0.1 IVB groups, IOP at 1 day after injection was significantly lower than before injection (P< 0.01, P = 0.03), and IOP at 30 days after injection was significantly higher than IOP at 1 day after injection (P < 0.01, P <0.01). In the 0.1 IVTA group, IOP at 30 days after injection was significantly higher than before injection (P = 0.01). Between groups, IOP at 1 day after injection in the 0.05 IVB group was significantly lower than IOP in the 0.1 IVTA group (P = 0.03).
Go to : 
References
1. Gordon MS, Cunnigham D. Managing patients treated with bavacizumab combination therapy. Oncology. 2005; 69:25–33.
2. Gunther JB, Altaweel MM. Bevacizumab (Avastin) for the treatment of ocular disease. Surv Ophthalmol. 2009; 54:372–400.

3. Kim TH, Moon YS, Chin HS. Change of residual period and clearance rate of intravitreal triamcinolone according to initial injection dosage. J Korean Ophthalmol Soc. 2005; 46:1569–74.
4. Jermak CM, Dellacroce JT, Heffez J, Peyman GA. Triamcinolone acetonide in ocular therapeutics. Surv Ophthalmol. 2007; 52:503–22.

5. Hollands H, Wong J, Bruen R, et al. Short-term intraocular pressure changes after intravitreal injection of bevacizumab. Can J Ophthalmol. 2007; 42:807–11.

6. Falkenstein IA, Cheng L, Freeman WR. Changes of intraocular pressure after intravitreal injection of bevacizumab (avastin). Retina. 2007; 27:1044–7.

7. Bakri SJ, Pulido JS, McCannel CA, et al. Immediate intraocular pressure changes following intravitreal injections of triamcinolone, pegaptanib, and bevacizumab. Eye. 2009; 23:181–5.

8. Özkiris A, Erkilic K. Complications of intravitreal injection of triamcinolone acetonide. Can J Ophthalmol. 2005; 40:63–8.
9. Jonas JB, Kreissig I, Degenring R. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol. 2003; 87:24–7.

10. Oh MJ, Choi KR, Lee SY, Lee JH. Clinical manifestation of intraocular pressure elevation after intravitreal injection of triamcinolone acetonide. J Korean Ophthalmol Soc. 2006; 47:1575–82.
11. Lee JM, Kim SJ, Yi KY, Kim HK. Intraocular pressure change after secondary intravitreal triamcinolone acetonide injection. J Korean Ophthalmol Soc. 2007; 48:97–102.
12. Park HY, Yi KY, Kim HK. Intravitreal pressure elevation after intravitreal triamcinolone acetonide injection. Korean J Ophthalmol. 2005; 19:122–7.
13. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997; 18:4–25.

14. Adamis AP, Aiello LP, D'Amato RA. Angiogenesis and ophthalmic disease. Angiogenesis. 1999; 3:9–14.
15. Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005; 25:111–8.

16. Kahook MY, Kimura AE, Wong LJ, et al. Sustained elevation in intraocular pressure associated with intravitreal bevacizumab injections. Ophthalmic Surg Lasers Imaging. 2009; 40:293–5.

17. Wiederholt M. Dirtect involvement of trabecular meshwork in the regulation of aqueous humor outflow. Curr Opin Ophthalmol. 1998; 9:46–9.
18. Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991; 43:109–42.
19. Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994; 63:175–95.

20. Brüne B, Knethen A, Sandau KB. Nitric oxide and its role in apoptosis. Eur J Pharmacol. 1998; 351:261–72.

21. Schuman JS, Erickson K, Nathanson JA. Nitrovasodilator effects on intraocular pressure and ocular facility in monkeys. Exp Eye Res. 1994; 58:99–105.
22. Wang RF, Podos SM. Effect of the topical application of nitro-glycerin on intraocular pressure in normal and glaucomatous monkeys. Exp Eye Res. 1995; 60:337–9.

23. Nathanson JA, McKee M. Alteration of ocular nitric oxide synthase in human glaucoma. Invest Ophthalmol Vis Sci. 1995; 36:1774–84.
24. Matsuo T. Basic nitric oxide production is enhanced by hydraulic pressure in cultured human trabecular cells. Br J Ophthalmol. 2000; 84:631–5.
25. Bouloumie A, Schini-Kerth VB, Busse R. Vascular endothelial growth factor up-regulates nitric oxide synthase expression in endothelial cells. Cardiovasc Res. 1999; 41:773–80.
26. Dulak J, Jozkowicz A, Dembinska-Kiec A, et al. Nitric oxide induces the synthesis of vascular endothelial growth factor by rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000; 20:659–66.

27. Wiederholt M, Sturm A, Lepple-Wienhues A. Relaxation of trabecular meshwork and ciliary muscle by release of nitric oxide. Invest Ophthalmol Vis Sci. 1994; 35:2515–20.
28. Spitzer MS, Yoeruek E, Sierra A, et al. Comparative anti-proliferative and cytotoxic profile of bevacizuman (Avastin), pegaptanib (macugen) and ranibizumab (Lucentis) on different ocular cells. Graefes Arch Clin Exp Ophthalmol. 2007; 245:1837–42.
29. Iriyama A, Chen Y-N, Tamaki Y, Yanagi Y. Effect of anti-VEGF on retinal gangion cells in rats. Br J Ophthalmol. 2007; 91:1230–3.
30. Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal bevacizumab (Avastin). Am J Ophthalmol. 2007; 114:855–9.

31. Kim SH, Kim JW. Effect of bevacizumab on survival and production of nitric oxide in trabecular meshwork cells. Ophthalmology. 2009; 50:1404–8.

32. Krohne TU, Eter N, Holz FG, Meyer CH. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am J Ophthalmol. 2008; 146:508–12.

33. Jones RF, Maurice DM. New method of measuring the rate of aqueous flow in man with fluorescein. Exp Eye Res. 1966; 5:208–10.
34. Polansky JR, Alvarado JA. Cellular mechanisms influencing the aqueous humor outflow pathway. Ophthalmology. 1987; 94:851–3.
35. Parshley DE, Bradley JBM, Samples JR, et al. Early changes in matrix metalloproteinases and inhibitors after in vitro laser treatment to the trabecular meshwork. Curr Eye Res. 1995; 14:537–44.
36. Parshley DE, Bradley JBM, Fisk A, et al. Laser trabeculoplasty induced stromelysin expression by trabecular juxtacanalicular cells. Invest Ophthalmol Vis Sci. 1996; 37:795–804.
37. Kahook MY, Ammar DA. In vitro effects of antivascular endothelial growth factors on cultured human trabecular meshwork cells. J Glaucoma. 2010; 19:437–41.

38. Yang YH, Kim KR, Yang SW, Yim HB. The effect of intravitreal triamcinolone acetonide on intraocular pressure. J Korean Ophthalmol Soc. 2004; 45:1081–5.
Go to : 
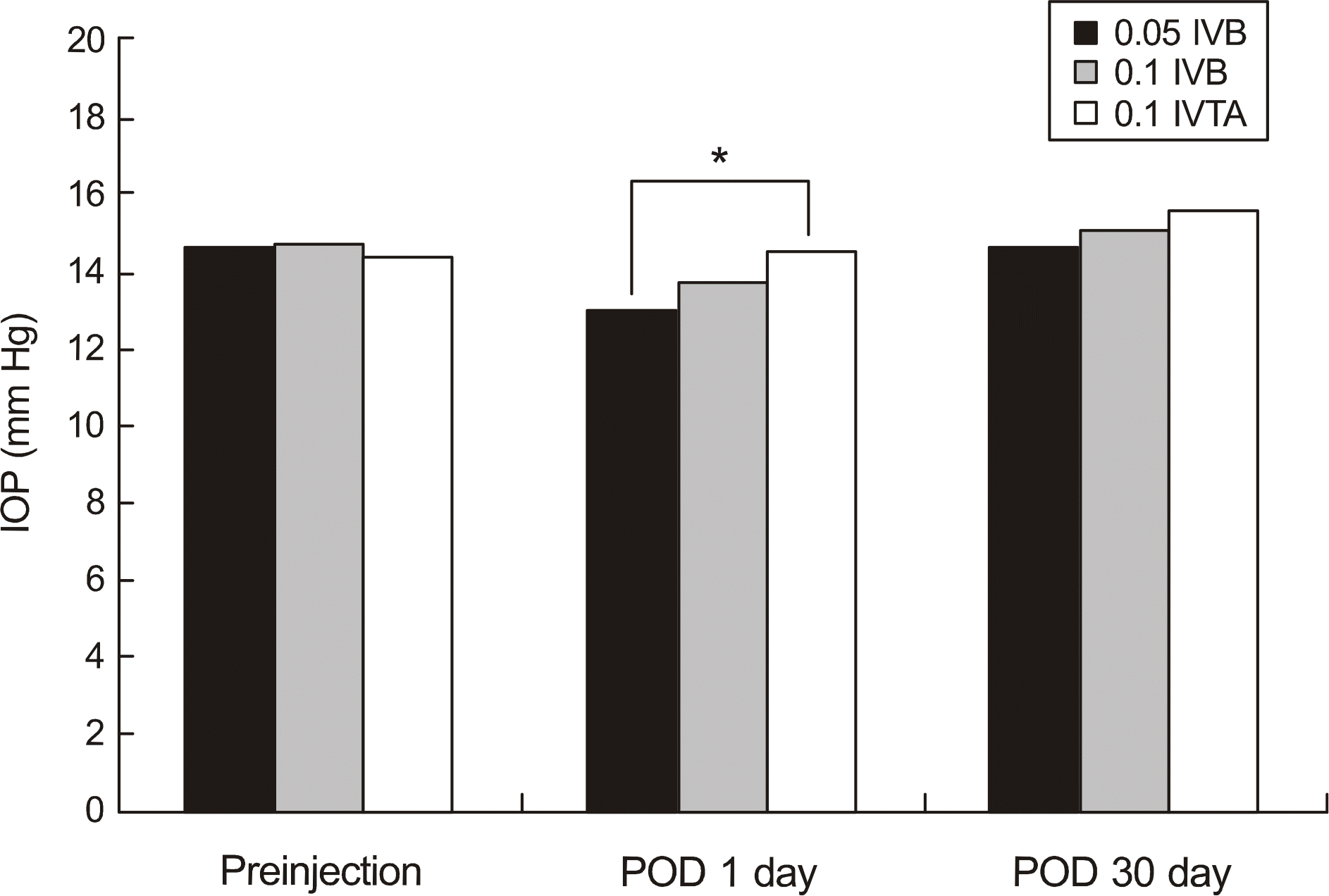 | Figure 1.Comparison of intraocular pressure between groups at the same time of injection. There was significant difference between groups at POD 1 day (ANOVA; P = 0.043).
* Indicates a statistically significant difference between 0.05 IVB group and 0.1 IVTA group at POD 1 day (Post hoc test; P = 0.033).
|
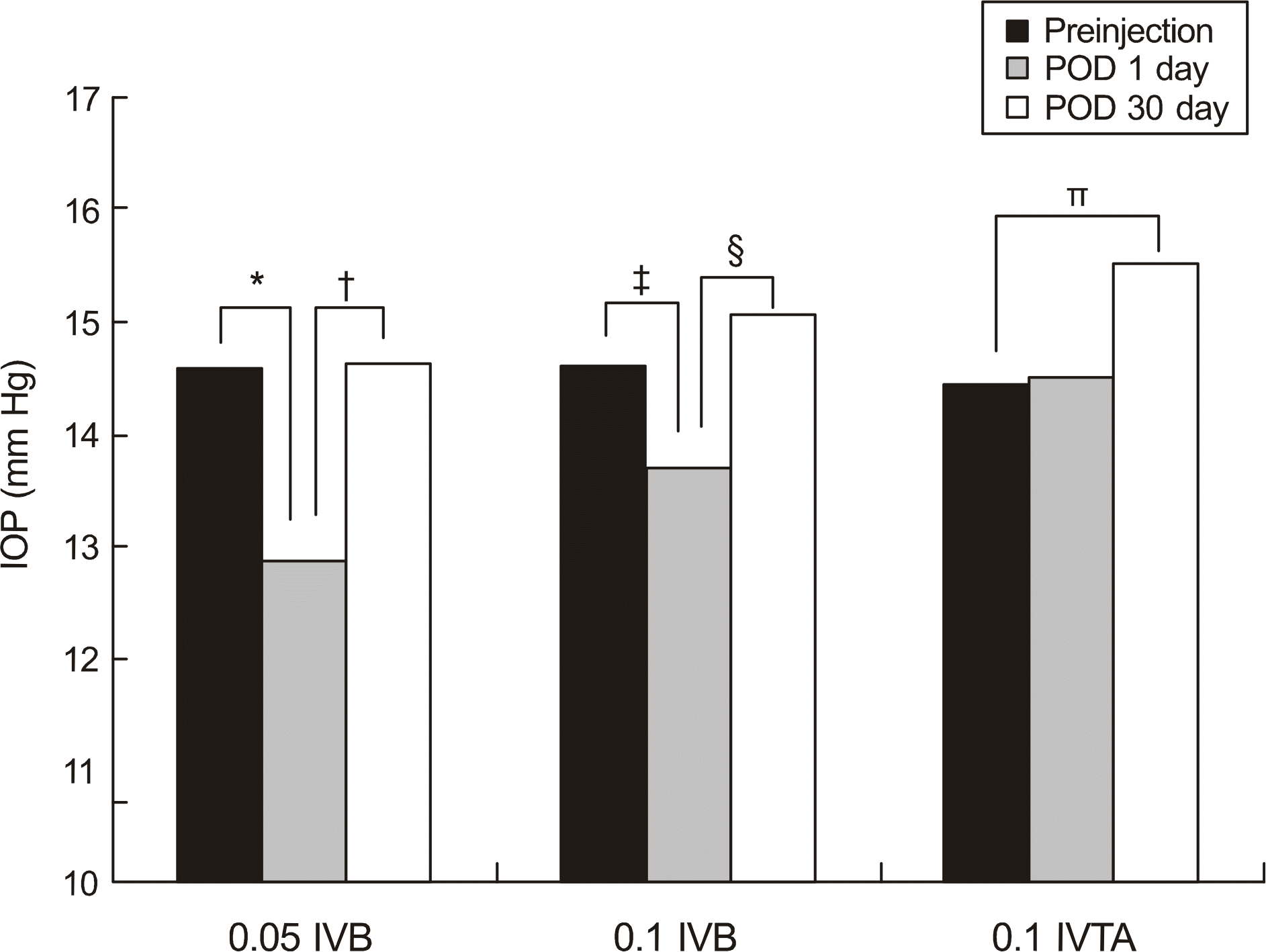 | Figure 2.Comparison of intraocular pressure within groups at different time of injection. Symbols denote statistically significant difference between the two groups.
* P< 0.001l; † P< 0.001l ‡ P = 0.035; § P = 0.008; ∏ P = 0.01.
|
Table 1.
Demographic features
| Factors | IVB group (n=40) | IVTA group (n=40) | P value |
|---|---|---|---|
| Sex | | | 0.445 |
| Male | 24 (54.5%) | 20 (50%) | |
| Female | 16 (44.4%) | 20 (50%) | |
| Age (year, mean ± SD) | 59.32 ± 13.92 | 59.37 ± 10.46 | 0.986 |
| IOP* (mmHg) | 14.62 ± 3.09 | 14.47 ± 2.88 | 0.823 |
| Diabetes mellitus | 11 (27.5%) | 29 (72.5%) | < 0.001 |
| Laterality | | | 0.535 |
| OD | 20 (50%) | 22 (55%) | |
| OS | 20 (50%) | 18 (45%) | |
| Diagnosis | | | < 0.001 |
| AMD† | 17 (42.5%) | 0 (0%) | |
| DMR‡ | 9 (22.5%) | 29 (72.5%) | |
| BRVO§ | 12 (30.0%) | 8 (20.0%) | |
| CRVO∏ | 2 (5.0%) | 3 (7.5%) | |
Table 2.
Characteristics of patients with higher IOP-lowering response patients in IVB 0.05 ml group
| Factors | Higher response patients (n=11) | Other patients (n=29) | P value |
|---|---|---|---|
| Sex | | | 0.312 |
| Male | 8 (72.7%) | 16 (55.2%) | |
| Female | 3 (27.3%) | 13 (44.8%) | |
| Age (year, mean ± SD) | 63.09 ± 11.64 | 57.89 ± 14.62 | 0.254 |
| IOP* before injection | 16.45 ± 3.07 | 13.93 ± 2.85 | 0.030# |
| IOP* at POD 1 day | 11.27 ± 2.19 | 13.51 ± 2.81 | 0.014# |
| IOP* at POD 30 day | 15.18 ± 2.40 | 14.48 ± 2.32 | 0.418 |
| Diabetes mellitus | 2 (18.2%) | 9 (31.0%) | 0.471 |
| Laterality | | | 0.288 |
| OD | 7 (63.6%) | 13 (44.8%) | |
| OS | 4 (36.4%) | 16 (55.2%) | |
| Diagnosis | | | 0.808 |
| AMD† | 4 (36.4%) | 13 (44.8%) | |
| DMR‡ | 2 (18.2%) | 7 (24.1%) | |
| BRVO§ | 4 (36.4%) | 8 (27.6%) | |
| CRVO∏ | 1 (9.1%) | 1 (3.4%) | |
Table 3.
Characteristics of patients with higher IOP-lowering response patients in IVB 0.1 ml group
| Factors | Higher response patients (n = 9) | Other patients (n = 31) | P value |
|---|---|---|---|
| Sex | | | 0.064 |
| Male | 8 (88.9%) | 16 (48.4%) | |
| Female | 1 (11.1%) | 15 (51.6%) | |
| Age (year, mean ± SD) | 57.77 ± 11.11 | 59.77 ± 14.77 | 0.667 |
| IOP* before injection | 15.66 ± 1.93 | 14.38 ± 2.38 | 0.119 |
| IOP* at POD 1 day | 11.22 ± 1.30 | 13.38 ± 2.96 | 0.041# |
| IOP* at POD 30 day | 14.00 ± 3.16 | 15.41 ± 3.54 | 0.268 |
| Diabetes mellitus | 2 (22.2%) | 9 (29.0%) | 0.687 |
| Laterality | | | 0.705 |
| OD | 5 (55.6%) | 15 (48.4%) | |
| OS | 4 (44.4%) | 16 (51.6%) | |
| Diagnosis | | | 0.159 |
| AMD† | 2 (22.2%) | 15 (48.4%) | |
| DMR‡ | 1 (11.1%) | 8 (25.8%) | |
| BRVO§ | 5 (55.6%) | 7 (22.6%) | |
| CRVO∏ | 1 (11.1%) | 1 (3.2%) | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download