Abstract
Purpose
To analyze the characteristics of anatomical non-response to intravitreal bevacizumab (IVB) in patients with neovascular age-related macular degeneration (AMD).
Methods
Neovascular AMD patients who were treated with IVB were studied. A non-responder was defined as a patient whose eyes had no change in choroidal neovascularization (CNV) lesion size or fluorescein leakage and no change in foveal thickness (FT) after at least two administrations of IVB. Demographic findings and efficacy outcomes were compared between responders and non-responders based on patient gender, age, visual acuity (VA), FT, CNV lesion type, CNV lesion size, presence of serous retinal detachment (SRD), presence of pigment epithelial detachment (PED), PED size, and presence of sub-macular hemorrhage (SMH).
Results
Five patients (six eyes; 13.6%) were identified as non-responders to treatment with IVB. The mean age of the non-responder group (75.17 ± 3.66 yers) was greater (p = 0.237) than that of the responder group (71.89 ± 8.06 years), and the proportion of occult CNV (85.7% versus 55.3%, p = 0.375) was higher in the non-responder group, although there was no significant difference compared with that of the responder group. The PED size of the non-responder group (4.42± 1.56 mm2) was significantly larger than that of the responder group (1.51 ± 2.33 mm2, p = 0.001).
Go to : 
References
1. Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992; 99:933–43.
2. Klein R, Davis MD, Magli YL, et al. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991; 98:1128–34.

3. Ferris FL 3rd. Senile macular degeneration: review of epidemiologic features. Am J Epidemiol. 1983; 118:132–51.

4. Ferris FL 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984; 102:1640–2.

5. Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005; 25:111–8.

6. Ng EW, Adamis AP. Targeting angiogenesis, the underlying disorder in neovascular age-related macular degeneration. Can J Ophthalmol. 2005; 40:352–68.

7. Kaiser PK, Brown DM, Zhang K, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007; 144:850–7.

8. Gragoudas ES, Adamis AP, Cunningham ET, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004; 351:2805–16.

9. Cleary CA, Jungkim S, Ravikumar K, et al. Intravitreal bevacizumab in the treatment of neovascular age-related macular degeneration, 6 and 9 month results. Eye. 2008; 22:82–6.
10. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005; 36:331–5.

11. Ferrara N, Damica L, Shams N, et al. Development of ranibizumab, an antivascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006; 26:859–70.

12. Lucio-Eterovic AK, Piao Y, de Groot JF. Mediators of glio-blastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res. 2009; 15:4589–99.

13. Cao Y, Zhong W, Sun Y. Improvement of antiangiogenic cancer therapy by understanding the mechanisms of angiogenic factor in-terplay and drug resistance. Semin Cancer Biol. 2009; 19:338–43.

14. Francia G, Emmenegger U, Kerbel RS. Tumor-associated fibroblasts as “Trojan Horse” mediators of resistance to anti-VEGF therapy. Cancer Cell. 2009; 15:3–5.

15. Crawford Y, Kasman I, Yu L, et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009; 15:21–34.

16. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004; 25:581–611.

17. Cho M, Barbazetto IA, Freund KB. Refractory neovascular agerelated macular degeneration secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol. 2009; 148:70–8.

18. Ghajarnia M, Kurup S, Eller A. The Therapeutic effects of intravitreal Bevacizumab in a patient with recalcitrant idiopathic polypoidal choroidal vasculopathy. Semin Ophthalmol. 2007; 22:127–31.

19. Gomi F, Sawa M, Sakaguchi H, et al. Efficacy of intravitreal bevacizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol. 2008; 92:70–3.

20. Ritter M, Bolz M, Sacu S, et al. Effect of intravitreal ranibizumab in avascular pigment epithelial detachment. Eye. 2010; 24:962–8.

21. Brancato R, Introini U, Bolognesi G, et al. ICGA-guided laser photocoagulation of occult choroidal neovascularization in age-related macular degeneration. Retina. 2000; 20:134–42.

22. el Matri L, Chebil A, Kort F, et al. Intravitreal injection of triamcinolone combined with bevacizumab for choroidal neovascularization associated with large retinal pigment epithelial detachment in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2010; 248:779–84.

23. Boyer DS, Antoszyk AN, Awh CC, et al. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007; 114:246–52.

24. Kaiser PK, Brown DM, Zhang K, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR result. Am J Ophthalmol. 2007; 144:850–7.
25. Azad RV, Khan MA, Chanana B, Azad S. Intravitreal bevacizumab for subfoveal choroidal neovascularization secondary to age-related macular degeneration in an Indian population. Jpn J Ophthalmol. 2008; 52:52–6.

26. Boyer DS, Antoszyk AN, Awh CC, et al. Subgroup analysis of the MARINA Study of Ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007; 114:246–52.

Go to : 
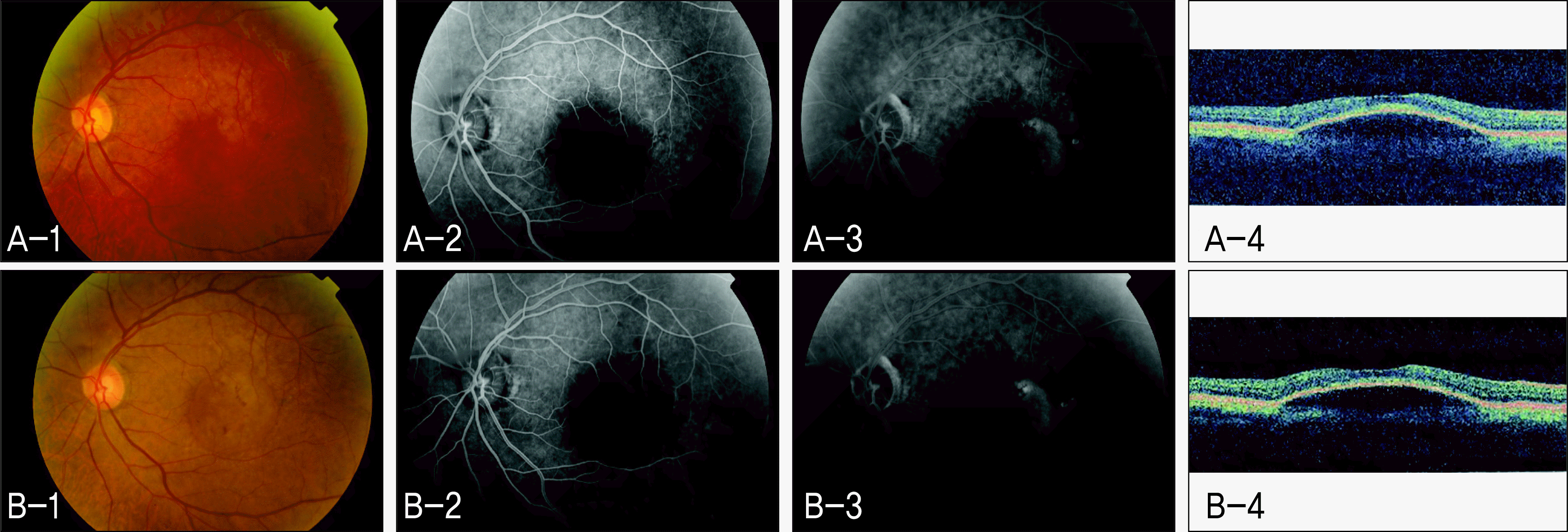 | Figure 1.Non-responder to intravitreal bevacizumab in Case 1 (Left eye). (A-1) Fundus photograph (FP) before intravitreal bevacizumab injection. (A-2) Early phase of fundus fluorescein angiography (FAG) before intravitreal bevacizumab injection. (A-3) Late phase of FAG before intravitreal bevacizumab injection. (A-4) Optical coherence tomography (OCT) scan before intravitreal bevacizumab injection. (B-1) FP 3 months after intravitreal bevacizumab injection. (B-2) Early phase of FAG 3 months after intravitreal bevacizumab injection. (B-3) Late phase of FAG 3 months after intravitreal bevacizumab injection. (B-4) OCT scan 3 months after intravitreal bevacizumab injection. |
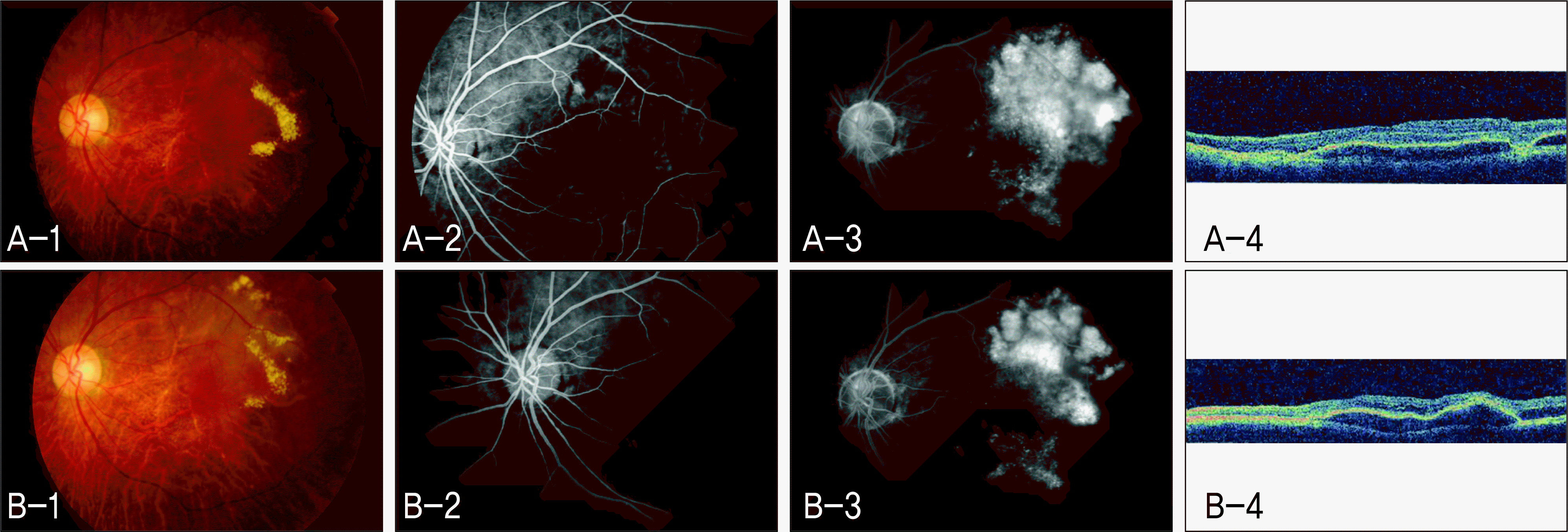 | Figure 2.Non-responder to intravitreal bevacizumab in Case 2 (Left eye). (A-1) Fundus photograph (FP) before intravitreal bevacizumab injection. (A-2) Early phase of fundus fluorescein angiography (FAG) before intravitreal bevacizumab injection. (A-3) Late phase of FAG before intravitreal bevacizumab injection. (A-4) Optical coherence tomography (OCT) scan before intravitreal bevacizumab injection. (B-1) FP 3 months after intravitreal bevacizumab injection. (B-2) Early phase of FAG 3 months after intravitreal bevacizumab injection. (B-3) Late phase of FAG 3 months after intravitreal bevacizumab injection. (B-4) OCT scan 3 months after intravitreal bevacizumab injection. |
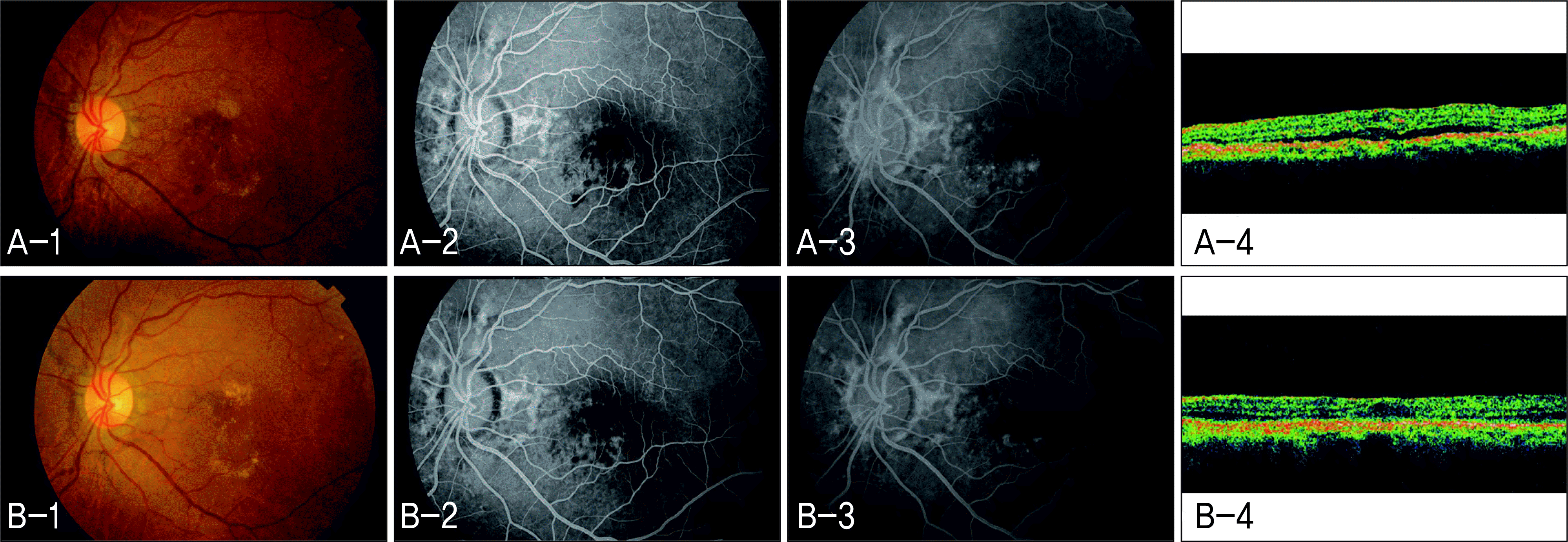 | Figure 3.Responder to intravitreal bevacizumab in Case 3 (Left eye). (A-1) Fundus photograph (FP) before intravitreal bevacizumab injection. (A-2) Early phase of fundus fluorescein angiography (FAG) before intravitreal bevacizumab injection. (A-3) Late phase of FAG before intravitreal bevacizumab injection. (A-4) Optical coherence tomography (OCT) scan before intravitreal bevacizumab injection. (B-1) FP 3 months after intravitreal bevacizumab injection. (B-2) Early phase of FAG 3 months after intravitreal bevacizumab injection. (B-3) Late phase of FAG 3 months after intravitreal bevacizumab injection. (B-4) OCT scan 3 months after intravitreal bevacizumab injection. |
Table 1.
Comparison of clinical and posterior retinal characteristics between responder and non-responder (Mean±SD)
| Variable | Responder (n=38) | Non-responder (n=6) | P value* |
|---|---|---|---|
| Age (yrs) | 71.89 ± 8.06 | 75.17 ± 3.66 | 0.237 |
| Gender (M:F) | 25: 13 | 3: 3 | 0.652 |
| Duration of F/U (mon) | 13.66 ± 11.37 | 16.17 ± 8.66 | 0.244 |
| OD: OS | 14: 24 | 3: 3 | 0.662 |
| Initial visual acuity | 0.27 ± 0.21 | 0.30 ± 0.23 | 0.744 |
| IOP† (mmHg) | 13.79 ± 3.35 | 14.67 ± 2.94 | 0.514 |
| SE‡ (D) | 0.38 ± 1.43 | −0.04 ± 1.58 | 0.595 |
| Number of injection | 2.26 ± 1.57 | 3.33 ± 1.63 | 0.081 |
| CNV§ lesion size (μ m) | 4668.37 ± 1990.80 | 4613.50 ± 2064.80 | 0.507 |
| Foveal thickness (μ m) | 272.44 ± 103.31 | 254.00 ± 167.34 | 0.328 |
| Macular volume (mm3) | 7.27 ± 1.01 | 7.87 ± 3.56 | 0.286 |
| Presence of SRD∏ (No:Yes) | 16: 22 | 4: 2 | 0.139 |
| SRD height (μ m) | 160.82 ± 112.14 | 336.36 | 0.180 |
| CNV lesion type (Predominantly classic: Occult) | 17: 21 | 1: 5 | 0.375 |
| Presence of PED# (No:Yes) | 5: 33 | 1: 5 | 0.609 |
| PED size (mm2) | 1.51 ± 2.33 | 4.42 ± 1.56 | 0.001 |
| Presence of SMH@ (No:Yes) | 20: 18 | 2: 4 | 0.664 |
Table 2.
Comparison of visual acuity, foveal thickness, macular volume between at baseline and at six months after treatment in each group (Mean ± SD).
| |
Responder (n=38) |
Non-responder (n=6) |
||||
|---|---|---|---|---|---|---|
| Baseline | At 6 mon | P value* | Baseline | At 6 mon | P value† | |
| Visual acuity | 0.27 ± 0.21 | 0.39 ± 0.27 | <0.001 | 0.30 ± 0.23 | 0.22 ± 0.23 | 0.109 |
| Foveal thickness (μ m) | 272.44 ± 103.31 | 194.19 ± 65.00 | 0.010 | 254.00 ± 167.34 | 337.80 ± 216.31 | 0.080 |
| Macular volume (mm3) | 7.27 ± 1.01 | 6.41 ± 0.65 | 0.002 | 7.87 ± 3.56 | 8.22 ± 3.50 | 0.080 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download