Abstract
Purpose
To evaluate the short-term effect of an intravitreal injection of ranibizumab in the treatment of diabetic macular edema.
Methods
Eighteen eyes of 18 patients who underwent intravitreal ranibizumab injection for the treatment of diabetic macular edema between March 1 and November 30, 2009 were retrospectively evaluated. Complete ophthalmic examinations including best corrected visual acuity and optical coherence tomography (OCT) were performed at baseline and follow-up visits at one and three months.
Results
The mean visual acuity improved from logMAR 0.74 ± 0.45 at baseline to logMAR 0.44 ± 0.26 at one month and to logMAR 0.42 ± 0.23 at three months (p < 0.05). The mean central macular thickness decreased from 429.5 ± 71.9 μ m at baseline to 299.9 ± 81.2 μ m at one month and to 284.6 ± 82.6 μ m at three months (p < 0.05). No adverse side effects were observed following the injections.
Go to : 
References
1. Ryan SJ. Nonproliferative diabetic retinopathy. Chew EY, Ferris FL III, editors. Retina. 4th ed.New York: Mosby, Elsevier Inc.;2006. 2:chap.p. 67.
3. Hardy RA, Crawford JB. Retina. Vaughn D, Asbury T, Riordan-Eva P, editors. General Ophthalmology. 15th ed.Stamford, CT: Appleton & Lange;1999. p. 178–99.
4. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. IV. Diabetic macular edema. Ophthalmology. 1984; 91:1464–74.
5. Early Treatment Diabetic Retinopathy Study research group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number. Arch Ophthalmol. 1985; 103:1796–805.
6. Nguyen QD, Shah SM, Van Anden E, et al. Supplemental oxygen improves diabetic macular edema: a pilot study. Invest Ophthalmol Vis Sci. 2004; 45:617–24.

7. Vinores SA, Youssri AI, Luna JD, et al. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathol. 1997; 12:99–109.
8. Antcliff RJ, Marshall J. The pathogenesis of edema in diabetic maculopathy. Semin Ophthalmol. 1999; 14:223–32.

9. Funatsu H, Yamashita H, Ikeda T, et al. Vitreous levels of inter-leukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2003; 110:1690–6.

10. Funatsu H, Yamashita H, Sakata K, et al. Vitreous levels of vascular endothelial growth factor and intercellular adhesion molecule 1 are related to diabetic macular edema. Ophthalmology. 2005; 112:806–16.

11. Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006; 26:275–8.

12. Haritoglou C, Kook D, Neubauer A, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006; 26:999–1005.

13. Presta LG, Chen H, O'Connor SJ, et al. Humanization of an antivascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997; 57:4593–9.
14. Fischer S, Renz D, Schaper W, Karliczek GF. In vitro effects of dexamethasone on hypoxia-induced hyperpermeability and expression of vascular endothelial growth factor. Eur J Pharmacol. 2001; 411:231–43.

15. Bandi N, Kompella UB. Budesonide reduces vascular endothelial growth factor secretion and expression in airway (Calu-1) and alveolar (A549) epithelial cells. Eur J Pharmacol. 2001; 425:109–16.

16. Oh SB, Moon JW, Kim HC. Comparison of effects of intravitreal triamcinolone and bevacizumab in the treatment of diabetic macular edema. J Korean Ophthalmol Soc. 2009; 50:1190–6.

17. Lee JW, Lee BH, Lim JH, Lee KW. Intravitreal triamcinolone versus bevacizumab for treatment of diabetic macular edema. J Korean Ophthalmol Soc. 2009; 50:1184–9.

18. Erol N, Topbas S, Sahin A. Retinal break following an intravitreal triamcinolone acetonide injection. Int Ophthalmol. 2005; 26:163–5.

19. Ung T, Williams CP, Canning CR. Globe rupture as a complication of intravitreal injection of triamcinolone. Eye. 2007; 21:423–4.

20. Sampat KM, Garg SJ. Complications of intravitreal injections. Curr Opin Ophthalmol. 2010; 21:178–83.

21. Toh T, Borthwick JH. Acute retinal necrosis post intravitreal injection of triamcinolone acetonide. Clin Experiment Ophthalmol. 2006; 34:380–2.

22. Gillies MC, Simpson JM, Billson FA, et al. Safety of an intravitreal injection of triamcinolone: results from a randomized clinical trial. Arch Ophthalmol. 2004; 122:336–40.
23. Jager RD, Aiello LP, Patel SC, et al. Risks of intravitreous injection: a comprehensive review. Retina. 2004; 24:676–98.

24. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004; 25:581–611.

25. Boulton M, Foreman D, Williams G, McLeod D. VEGF localisation in diabetic retinopathy. Br J Ophthalmol. 1998; 82:561–8.

26. Campochiaro PA. C99-PKC412–003 Study Group. Reduction of diabetic macular edema by oral administration of the kinase inhibitor PKC412. Invest Ophthalmol Vis Sci. 2004; 45:922–31.

27. Chun DW, Heier JS, Topping TM, et al. A pilot study of multiple intravitreal injections of ranibizumab in patients with center-in-volving clinically significant diabetic macular edema. Ophthalmology. 2006; 113:1706–12.

28. Nguyen QD, Tatlipinar S, Shah SM, et al. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol. 2006; 142:961–9.

29. Nguyen QD, Shah SM, Heier JS, et al. Primary end point (Six months) results of the Ranibizumab for edema of the macular in diabetes (READ-2) study. Ophthalmology. 2009; 116:2175–81.
30. Fung AE, Rosenfeld PJ, Reichel E. The International Intravitreal Bevacizumab Safety Survey: using the internet to assess drug safety worldwide. Br J Ophthalmol. 2006; 90:1344–9.

31. Jonas JB, Spandau UH, Schlichtenbrede F. Short-term complications of intravitreal injections of triamcinolone and bevacizumab. Eye. 2008; 22:590–1.

32. Apte RS. Retinal pigment epithelial tear after intravitreal ranibizumab for subfoveal CNV secondary to AMD. Int Ophthalmol. 2007; 27:59–61.

33. Smith BT, Kraus CL, Apte RS. Retinal pigment epithelial tears in ranibizumab-treated eyes. Retina. 2009; 29:335–9.

34. Angulo Bocco MC, Glacet-Bernard A, Zourdani A, et al. [Intravitreous injection: retrospective study on 2028 injections and their side effects] J Fr Ophthalmol. 2008; 31:693–8.
35. Ladas ID, Karagiannis DA, Rouvas AA, et al. Safety of repeat intravitreal injections of bevacizumab versus ranibizumab: our experience after 2,000 injections. Retina. 2009; 29:313–8.
36. Stewart MW. Predicted biologic activity of intravitreal aberrations. Retina. 2007; 27:1196–200.
37. Kim SJ, Park YM, Lee SU, et al. Short-term safety and efficacy of intravitreal Bavacizumab injection. J Korean Ophthalmol Soc. 2009; 50:219–26.

38. Pieramici DJ, Rabena MD. Anti-VEGF therapy: comparison of current and future agents. Eye. 2008; 22:1330–6.

39. Gharbiya M, Giustolisi R, Allievi F, et al. Choroidal aberrations in pathologic myopia: intravitreal Ranibizumab versus Bevacizumab— a randomized controlled trial. Am J Ophthalmol. 2010; 149:458–64.
Go to : 
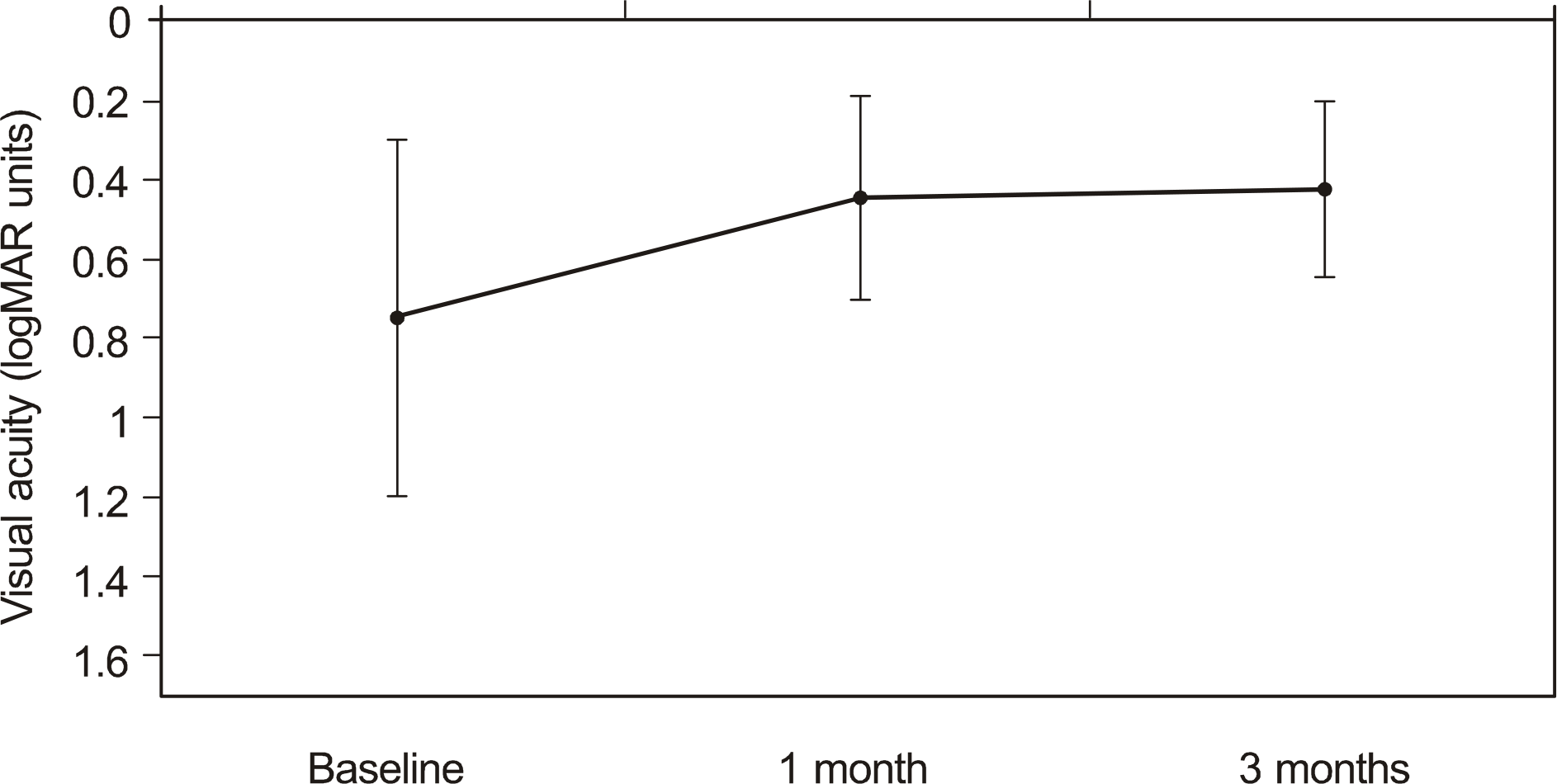 | Figure 1.A change of the visual acuity (logMAR) after ranibizumab injection. Mean visual acuity prior to injection was log MAR 0.74 ± 0.45. Visual acuity increased to logMAR 0.44± 0.26, log MAR 0.42 ± 0.23, 1 and 3 months after ranibizumab injection. |
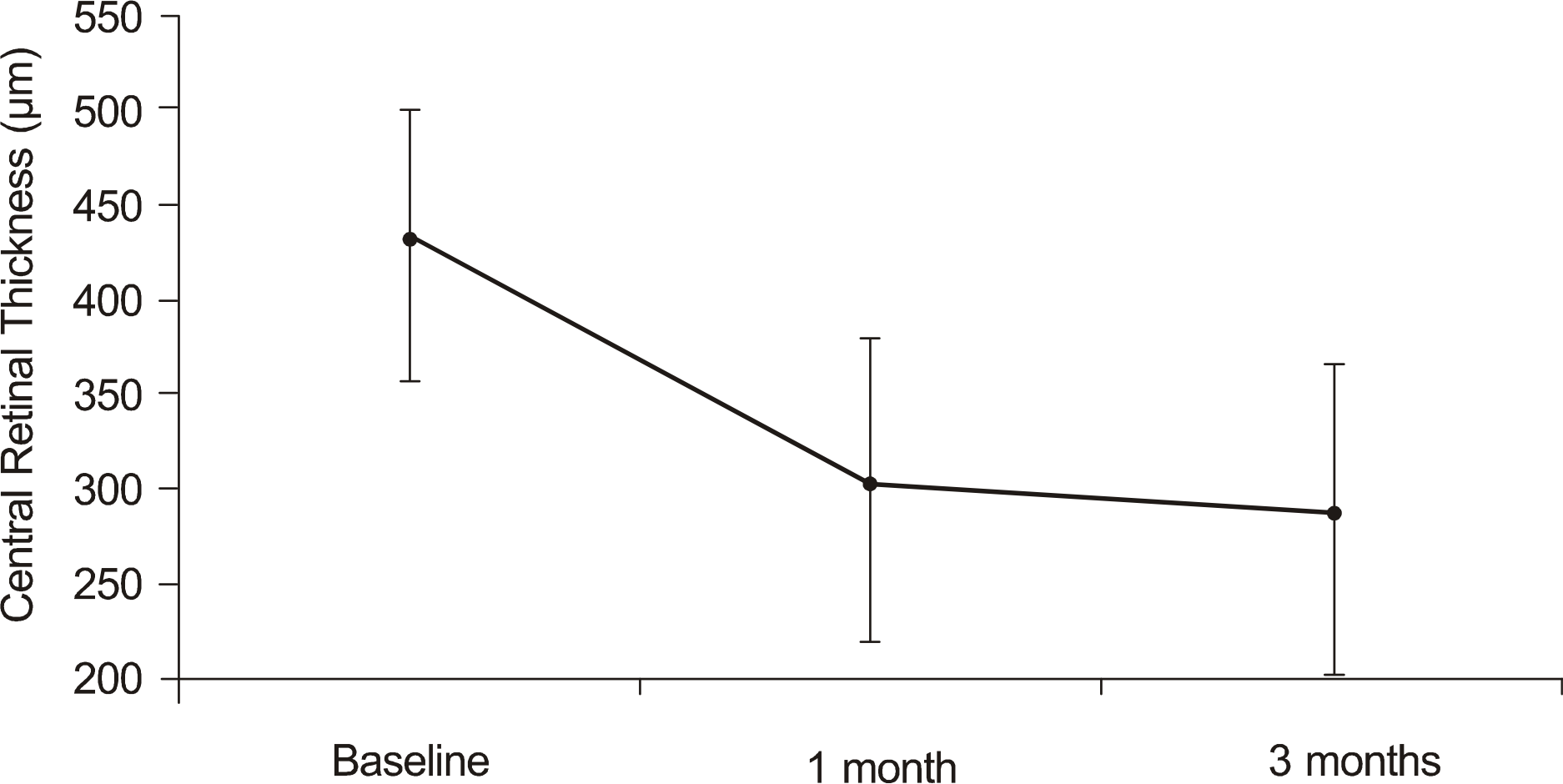 | Figure 2.A change in the central macular thickness after ranibizumab injection. Mean central macular thickness prior to injection was 429.5 ± 71.9 μ m. Central macular thickness decreased to 299.9 ± 81.2 μ m, 284.6 ± 82.6 μ m, 1 and 3 months after ranibizumab injection. |
Table 1.
Demographic data for all patients enrolled in the study
| Demographic data | |
|---|---|
| Number of eyes | 18 (10 right, 8 left) |
| Mean patient age (mean ± SD*) | 60.9 years (± 7.3) |
| Gender | 10 male, 8 female |
| Preoperative BCVA† (logMAR‡) | 0.74 ± 0.45 |
| Preoperative CMT§ | 429.5 ± 71.9 |
Table 2.
Changes in visual acuity and central retinal thickness after ranibizumab injection
| | Baseline | 1 month | 3 months |
|---|---|---|---|
| BCVA* | 0.74 ± 0.45 | 0.44 ± 0.26 | 0.42 ± 0.23 |
| (logMAR†, mean ± SD‡) | | (p=0.001) | (p<0.001) |
| CMT§ | 429.5 ± 71.9 | 299.9 ± 81.2 | 284.6 ± 82.6 |
| (μ m, mean ± SD) | | (p<0.001) | (p<0.001) |
Table 3.
Changes in number of eyes of visual acuity after intravitreal ranibizumab injection
| BCVA* (logMAR†) | Baseline | 1 month | 3 months |
|---|---|---|---|
| 0 ∼ 0.4 | 4 (22.2%) | 10 (55.6%) | 12 (66.7%) |
| 0.5 ∼ 0.9 | 8 (44.4%) | 7 (38.9%) | 5 (27.8%) |
| 1.0 or more | 6 (33.4%) | 1 (5.5%) | 1 (5.5%) |
Table 4.
Changes in number of eyes of central retinal thickness after intravitreal ranibizumab injection
| CMT* (μ m) | Baseline | 1 month | 3 months |
|---|---|---|---|
| Less than 300 | 0 | 8 (44.5%) | 13 (72.3%) |
| 300 ∼ 399 | 7 (38.9%) | 8 (44.5%) | 4 (22.2%) |
| 400 ∼ 499 | 7 (38.9%) | 1 (5.5%) | 0 |
| 500 or more | 4 (22.2%) | 1 (5.5%) | 1 (5.5%) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download