Abstract
Purpose
Chemical lights, also called Luminous Sticks, consist of a solution of diphenyl oxalate (C14H10O4) and hydrogen peroxide (H2O2). Human tissue can be damaged when the mixed solution contacts the human body. The authors report a single case of chemical injury of keratoconjunctiva by exposure to chemical lights.
Case summary
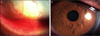
A 47-year-old man's right eye accidentally contacted the fluorescent material when breaking a Luminous Stick 7 days before being referred to our clinic. He had pain in the right eye and experienced visual loss. The patient's best corrected visual acuity in the right eye was 20/50. An ulcerative lesion with edema at the inferior bulbar and palpebral conjunctiva and coneal epithelial defect was observed upon biomicroscopic examination. The patient was hospitalized and antibiotics, steroids, mydriatic and artificial tear eye drops were applied for treatment. After 9 days of treatment, the best corrected visual acuity of the patient recovered to 20/20, and the conjunctiva and cornea were mostly healed. No complication was observed.
Conclusions
Chemical lights are commonly used in concerts and festivals. If the contents contact the eyes when breaking he chemical lights, various chemical burns can occur and cause ophthalmologic complications. Since no regulations have been passed regarding chemical lights, safety education and supervision are considered to be necessary for children.
Figures and Tables
Figure 1
Chemical light sticks are flexible plastic filled with hydrogen peroxide. Inside the tube is another smaller glass tube filled with diphenyloxalate

References
1. Jones NP, Hayward JM, Khaw PT, et al. Function of an ophthalmic "accident and emergency" department: results of a six month survey. Br Med J. 1986. 292:188–190.
2. Vernon SA. Analysis of all new cases seen in a busy regional center ophthalmic casualty department during a 24-week period. J R Soc Med. 1983. 76:279–282.
3. Pfister RR. Chemical injuries of the eye. Ophthalmology. 1983. 90:1246–1253.
4. Hong YJ, Kim DI, Kim HK. A Statistical Observation of Industrial Eye Injuries. J Korean Ophthalmol Soc. 1982. 23:627–633.
5. Kim SS, Yoo JM. A Clinical Study of Industrial Ocular Injuries. J Korean Ophthalmol Soc. 1988. 29:382–393.
6. Shon OO, Kim YJ. An Epidemiological Study of Occupational Ocular Injuries. J Korean Ophthalmol Soc. 1985. 26:525–531.
7. Saari KM, Leinonen J, Aine E. Management of chemical eye injuries with prolonged irrigation. Acta Ophthalmol. 1984. 161:52–59.
8. Pfister RR. Chemical injuries of the eye. Ophthalmology. 1983. 90:1246–1253.
9. Lazarus GS, Brown RS, Daniels JR, Fullmer HM. Human granulocyte collagenase. Science. 1968. 159:1483–1485.
10. Gnäadinger MC, Itoi M, Slansky HH, Dohlman CH. The role of collagenase in the alkali-burned cornea. Am J Ophthalmol. 1969. 68:478–483.
11. Friedenwald Js, Hughes WF, Hermann H. Acid burns of the eye. Arch Ophthalmol. 1946. 35:98–108.
12. Michael MR. Chemiluminescence from concerted peroxide decomposition reactions. Accounts of Chemical Research. 1969. 2:80–87.
13. Ingridet CM, Abigail B, Dominique AW, et al. Effects of phenol on barrier function of a human intestinal epithelial cell linecorrelate with altered tight junction protein
localization. Toxicol Appl Pharmacol. 2009. 241:61–70.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download