Abstract
Methods
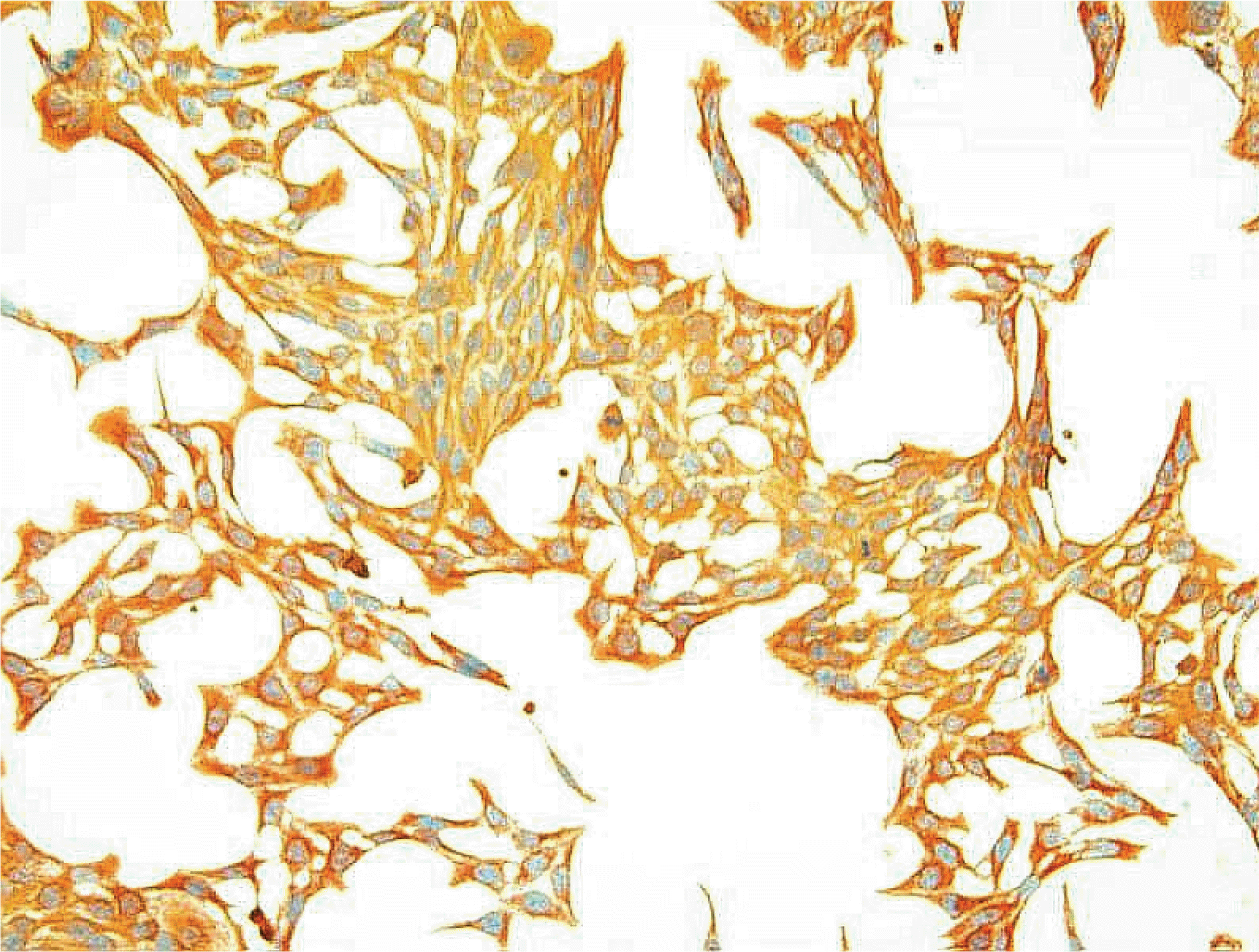
After immunostaining for GFAP, vimentin, S-100, and neurofilament, R28 cells were exposed to S-nitroso-N-acetyl-D, L-penicillamine (SNAP) at various concentrations, with and without the NO inhibitor, Nω-Nitro-L-arginine methyl ester (L-NAME) for 1 and 3 days. Cellular survival of R28 cells and the production of NO were quantified by rapid colorimetric assays using the MTT and Griess assay, respectively. To evaluate the effect of serum, 10% serum or serum-free media were used separately.
Results
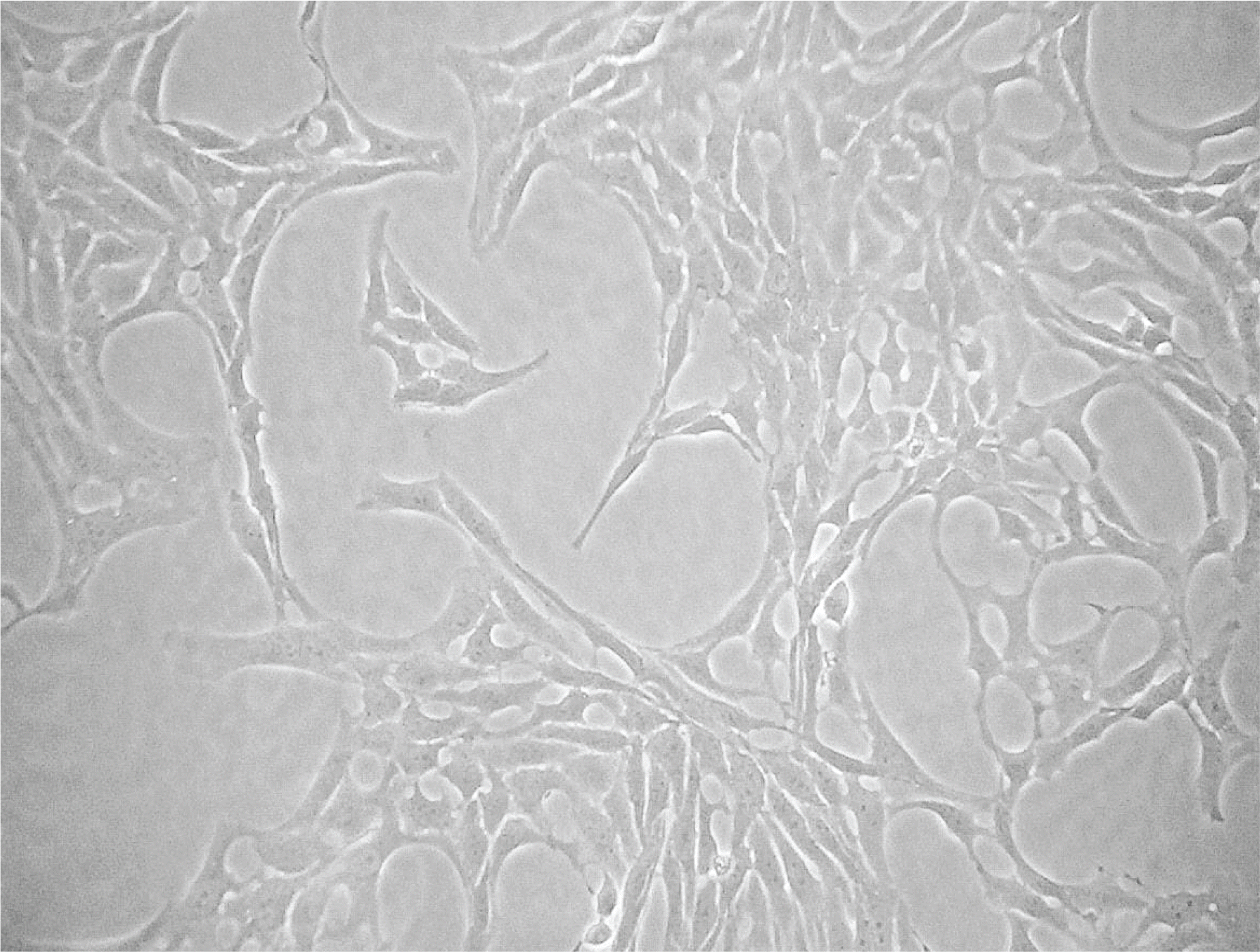
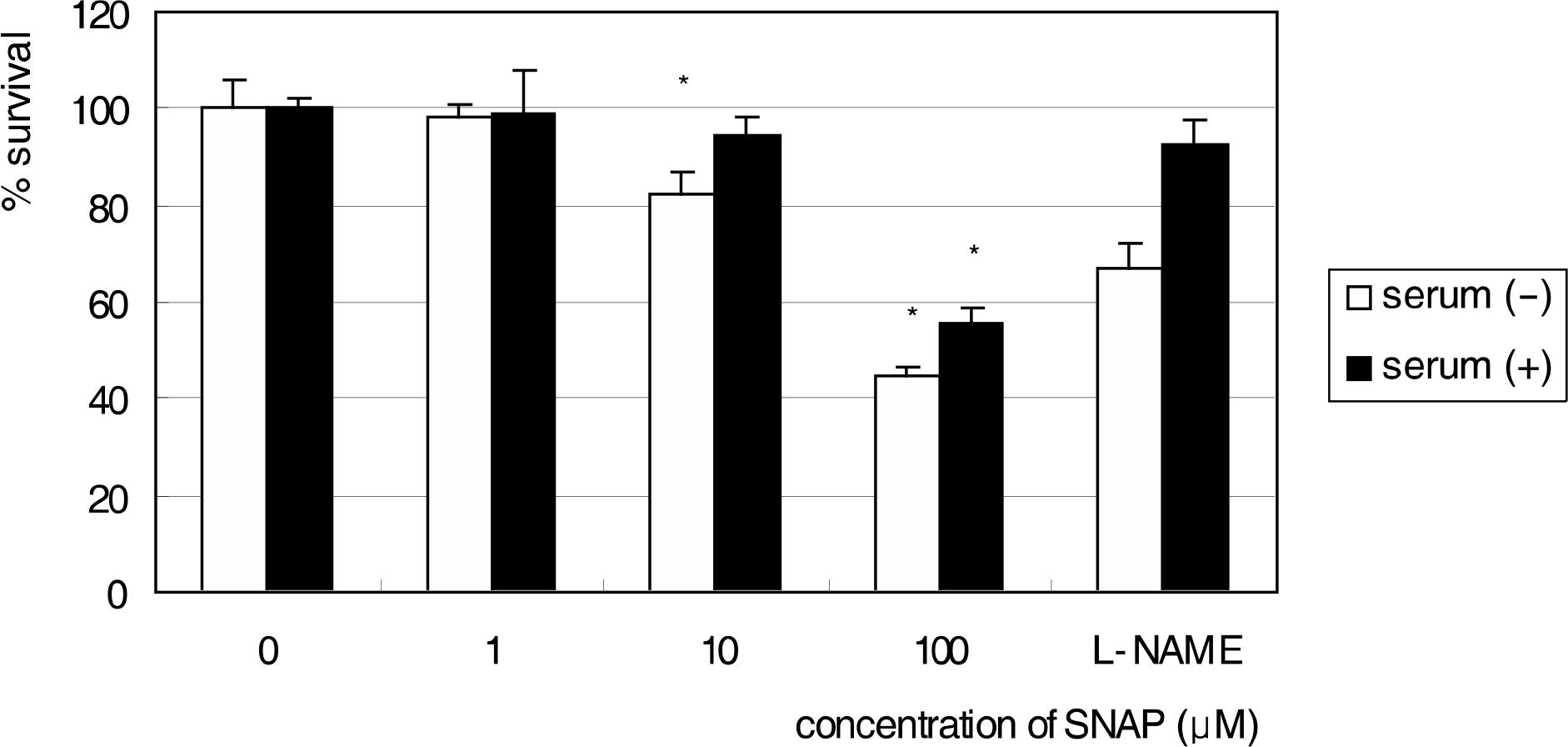
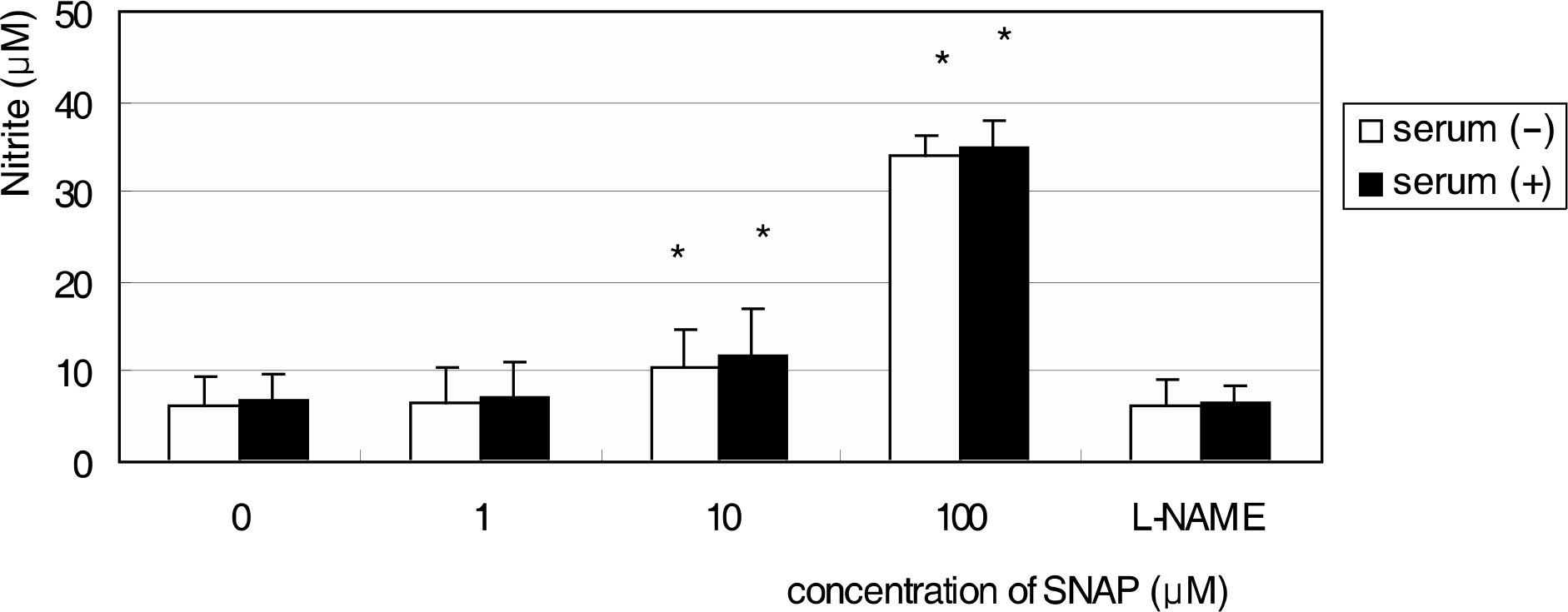
R28 cells showed strong immunoreactivity to GFAP and vimentin compared to S-100 or neurofilament. SNAP inhibited the survival of R28 cells in a dose-dependent manner, and this effect of NO on the cellular survival was abolished by L-NAME. These results were similar after exposure for 1 and 3 days, regardless of the presence of serum in the media.
Go to : 
References
1. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991; 43:109–42.
2. Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994; 63:175–95.

3. Brüne B, Knethen A, Sandau KB. Nitric oxide and its role in apoptosis. Eur J Pharmacol. 1998; 351:261–72.

4. Beck KF, Eberhardt W, Frank S, et al. Inducible NO synthase: Role in cell signaling. J Exp Biol. 1999; 202:645–53.
5. Becquet F, Courtois Y, Goureau O. Nitric oxide in the eye: multifaceted roles and diverse outcomes. Surv Ophthalmol. 1997; 42:71–82.

6. Meyer P, Champion C, Schlotzer-Schrehardt U, et al. Localization of nitric oxide synthase isoforms in porcine ocular tissues. Curr Eye Res. 1999; 18:375–80.

7. Geyer O, Podos SM, Mittag T. Nitric oxide synthase activity in tissues of the bovine eyes. Graefes Arch Clin Exp Ophthalmol. 1997; 235:786–93.
8. Nathanson JA, McKee M. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow pathway. Invest Ophthalmol Vis Sci. 1995; 36:1765–73.
9. Quigley HA, Nickells RW, Kerrigan LA, et al. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995; 36:774–86.
10. Otori Y, Wei J-Y, Barnstable CJ. Neurotoxic effects of low doses of glutamate on purified rat retinal ganglioncells. Invest Ophthalmol Vis Sci. 1998; 39:972–81.
11. Morgan J, Caprioli J, Koseki Y. Nitric oxide mediates excitotoxic and anoxic damage in rat retinal ganglion cells cocultured with astroglia. Arch Ophthalmol. 1999; 117:1524–9.

12. Vorwerk CK, Hyman BT, Miller JW, et al. The role of neuronal and endothelial nitric oxide synthase in retinal excitotoxicity. Invest Ophthalmol Vis Sci. 1997; 38:2038–44.
13. Seigel GM. The golden age of retinal cell culture. Mol Vis. 1999; 5:4.
14. Seigel GM, Mutchler AL, Imperato EL. Expression of glial markers in retinal precusor cell line. Mol Vis. 1996; 2:2.
15. Green LC, Wagner DA, Glogoski J, et al. Analysis of nitrate, nitrite and [15N]nitrate in biologic fluids. Anal Biochem. 1982; 126:131–8.
16. Nathanson JA, McKee M. Alterations of ocular nitric oxide synthase in human glaucoma. Invest Ophthalmol Vis Sci. 1995; 36:1774–84.
17. Neufeld AH, Hernandez MR, Gonzalez M. Nitric oxide synthase in the human glaucomatous optic nerve head. Arch Ophthalmol. 1997; 115:497–503.

18. Hu DN, Ritch R. Tissue culture of adult human retinal ganglion cells. J Glaucoma. 1997; 6:37–43.

19. Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988; 1:791–803.

20. Seigel GM, Takahashi M, Adamus G, McDaniel T. Intraocular transplantation of E1A-immortalized retinal precusor cells. Cell Transplant. 1998; 7:559–66.
21. Seigel GM, Chiu L, Paxhia A. Inhibition of neuroretinal cell death by insulinlike growth factor-1 and its analogs. Mol Vis. 2000; 6:157–63.
22. Barber AJ, Nakamura M, Wolpert EB, et al. Insulin rescues retinal neurons from apoptosis by a phosphatidyl 3-kinase/Akt-mediated mechanism that reduces the activation of Caspases-3. J Biol Chem. 2001; 276:32814–21.
23. Nakamura M, Barber AJ, Antonetti DA, et al. Excessive hexo-samines block the neuroprotective effect of insulin and induce apoptosis in retinal neurons. J Biol Chem. 2001; 276:43748–55.

24. Sun W, Seigel GM, Salvi RJ. Retinal precusor cells express functional ionotropic glutamate and GABA receptors. Neuroreport. 2002; 13:2421–4.
25. Kawasaki A, Otori Y, Barnstable CJ. Müller cell protection of rat retinal ganglion cells from glutamate and nitric oxide neurotoxicity. Invest Ophthalmol Vis Sci. 2000; 41:3444–50.
Go to : 
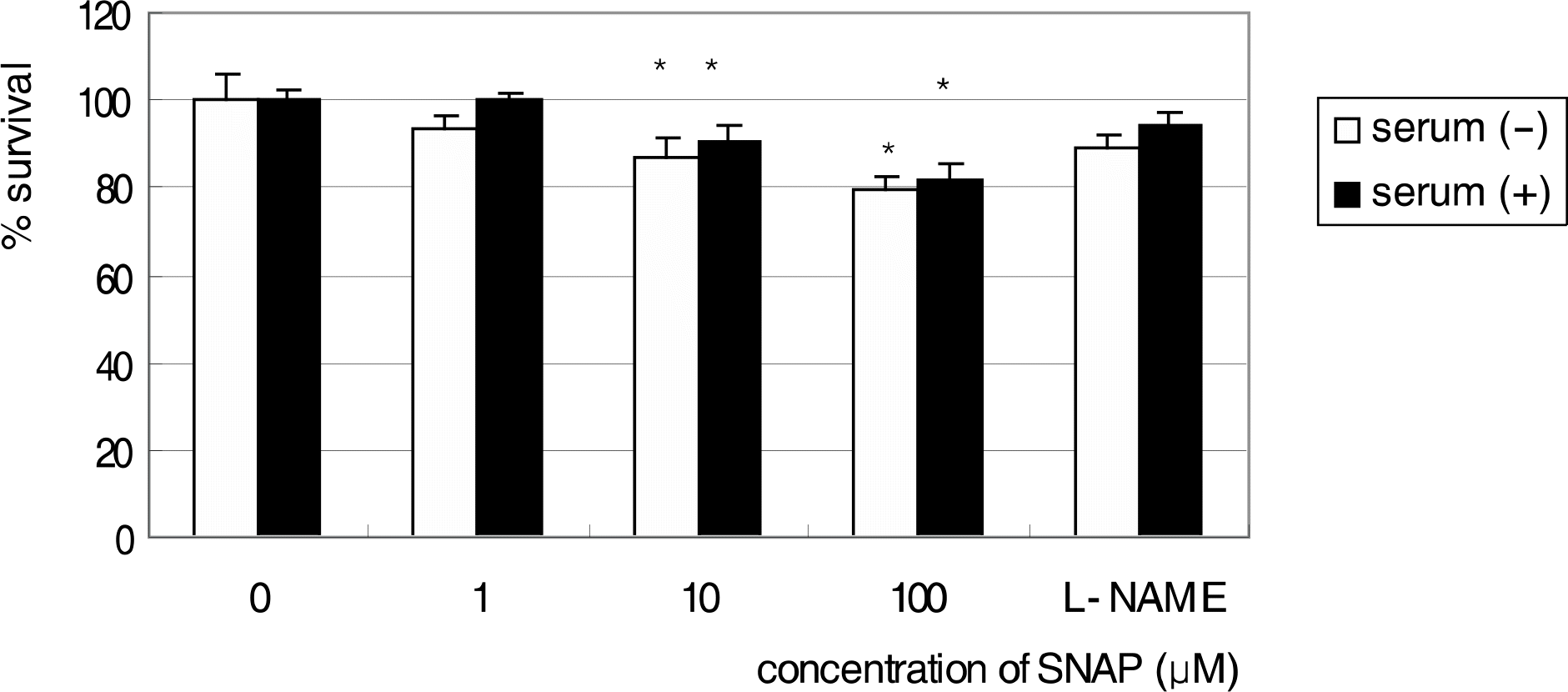 | Figure 3.Survival of R28 cells after exposed to SNAP for 1 day in serum-free and 10% serum-containing media. Cellular survival was decreased significantly with exposure to NO in a dose-dependent manner (* p<0.05). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download