Abstract
Purpose
The aim of the present study was to quantify and compare the vitreolytic effect of plasmin, hyaluronidase, and a combination of the two.
Methods
Thirty-six rabbits were randomized into 3 groups: (A) twelve rabbits had an intravitreal injection of plasmin 1 U with hyaluronidase 10 U/0.1 mL into the right eye, (B) twelve rabbits had an injection of plasmin alone (1 U/0.1 mL), and (C) twelve rabbits had an injection of hyaluronidase alone (10 U/0.1 mL). The left eye of each rabbit was used as control, which was injected with 0.1 mL phosphate buffered saline (PBS). The eyes were enucleated 1 hour and 24 hours after injection. The volume of fluid-type vitreous and gel-type vitreous was measured with a micropipette using the melting point as the difference. Statistical analysis was performed and light microscopy was used to assess potential damage to the retinal tissue.
Results
The volume of remaining gel-type vitreous was measured as 52.5%, 60.3%, 59.2%, and 76.5% after 1 hour enucleation and as 44.6%, 56.7%, 56.1%, and 74.7%, after 24 hours enucleation in group A, B, C, and control group, respectively. Group A, B, and C showed statistically significant differences against the control group. Group A (plasmin with hyaluronidase) showed less remaining gel-type vitreous volume than a single injection of plasmin or hyaluronidase alone.
Go to : 
References
2. Sebag J. Pharmacologic vitreolysis brewing? Retina. 2002; 22:1–3.
3. Hayreh SS, Jonas JB. Posterior Vitreous detachment: Clinical correlations. Ophthalmologica. 2004; 218:333–43.

5. Takahashi MK, Hikichi T, Akiba J, et al. Role of the vitreous and macular edema in branch retinal vein occlusion. Ophthalmic Surg Lasers. 1997; 28:294–9.
6. Sonoda K, Sakamoto T, Enaida H, et al. Residual vitreous cortex after surgical posterior vitreous separation visualized by intravitreal triamcinolone acetonide. Ophthalmology. 2004; 111:226–30.
7. Asami T, Terasaki H, Kachi S, et al. Ultrastructure of inter-nallimiting membrane removed during plasmin assisted vitrectomy from eyes with diabetic macular edema. Ophthalmology. 2004; 111:231–7.
8. Verstraeten TC, Chapman C, Hartzer M, et al. Pharmacologic inductionof posterior vitreous detachment in the rabbit. Arch Ophthalmol. 1993; 111:849–54.
10. Bishop PN. Structural macromolecules and supramolecular organisation of the vitreous gel. Prog-Retin Eye Res. 2000; 19:323–44.

11. Kuppermann BD, Thomas EL, de Smet MD, Grillone LR. Safety results of two phaseⅢ trials of an intravitreous injection of highly purified ovine hyaluronidase (Vitrase) for the management of vitreous hemorrhage. Am J Ophthalmol. 2005; 140:585–97.
12. Kim NJ, Yu HG, Yu YS, Chung H. Long term effect of plasmin on the vitreolysis in the rabbit eyes. Korean J Ophthalmology. 2004; 18:35–40.
13. Wang ZL, Zhang X, Xu X, et al. PVD following plasmin but nothyaluronidase: implication for combination pharmacologic vitreolysis. Retina. 2005; 25:38–43.
14. Staubach F, Nober V, Janknecht P. Enzyme-assisted vitrectomy in enucleated pig eyes: a comparison of hyaluronidase, chondroitinase, and plasmin. Curr Eye Res. 2004; 29:261–8.

15. Wang ZL, Zhang X, Xu X, et al. Pharmacologic vitreolysis combining the two enzymes plasmin and hyaluronidase. Retina. 2005; 25:674–5.
16. Howard M, Sen HA, Capoor S, et al. Measurement of adenosine concentration in aqueous and vitreous. Invest Ophthalmol Vis Sci. 1998; 39:1942–6.
17. Kim DS, Moon SW, Yu SY, Kwak HW. The structure of the internal limiting membrane removed by vitrectomy using tissue plasminogen activator. J Korean Ophthalmol Soc. 2008; 49:917–24.

18. Gottlieb JL, Antoszyk AN, Hatchell DL, Saloupis P. The safety of intravitreal hyaluronidase. A clinical and histological study. Invest Ophthalmol Vis Sci. 1990; 31:2345–52.
19. Harooni M, McMillan T, Refojo M. Efficacy and safety of enzymatic posterior vitreous detachment by intravitreal injection of hyaluronidase. Retina. 1998; 18:16–22.

20. Kang SW, Hyung S, Choi MY, Lee J. Induction of vitreolysis and vitreous detachment with hyaluronidase and perfluoropropane gas. Korean J Ophthalmol. 1995; 9:69–78.

21. Li X, Shi X, Fan J. Posterior vitreous detachment with plasmin in the isolated human eye. Graefes Arch Clin Exp Ophthalmol. 2002; 240:56–62.

22. Wang F, Wang Z, Sun X, et al. Safety and efficacy of dispase and plasmin in pharmacologic vitreolysis. Invest Ophthalmol Vis Sci. 2004; 45:3286–90.

23. Gandorfer A, Putz E, Welge-Lussen U, et al. Ultrastructure of the vitreoretinal interface following plasmin assisted vitrectomy. Br J Ophthalmol. 2001; 85:6–10.

24. Gandorfer A, priglinger S, Schebitz K, et al. Vitreoretinal morphology of plasmin-treated human eyes. Am J Ophthalmol. 2001; 133:156–9.
25. Gandorfer A, Rohleder M, Sethi C, et al. Posterior vitreous detachment induced by microplasmin. Invest Ophthalmol Vis Sci. 2004; 45:641–7.

26. Plantner JJ, Smine A, Quinn TA. Matrix metalloproteinases and metalloproteinase inhibitors in human interphotoreceptor matrix and vitreous. Curr Eye Res. 1998; 17:132–40.

27. Baramova EN, Bajou K, Remacle A, et al. Involvement of PA/plasminsystem in the processing of pro-MMP-9 and in the second step of pro-MMP-2 activation. FEBS Lett. 1997; 157:157–62.
28. Ramos-DeSimone N, Hahn-Dantona E, Sipley J, et al. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/ stromelysin-1 cascade enhances tumor cell invasion. J Biol Chem. 1999; 274:13066–76.
Go to : 
 | Figure 1.Photographs of the experimental process. (A, B and C) Peeling the sclera, choroid, retina tissue of frozen rabbit eyeball. (D, E) Removal of the cornea, aqueous humor, iris and lens of frozen eyeball. (F) Separated frozen vitreous, lens and iris respectively (Blue asterisk: vitreous green asterisk: lens white asterisk: iris). (G, H and I) Melting the vitreous at room temperature (red arrow: gel type vitreous yellow arrow: fluid type vitreous). |
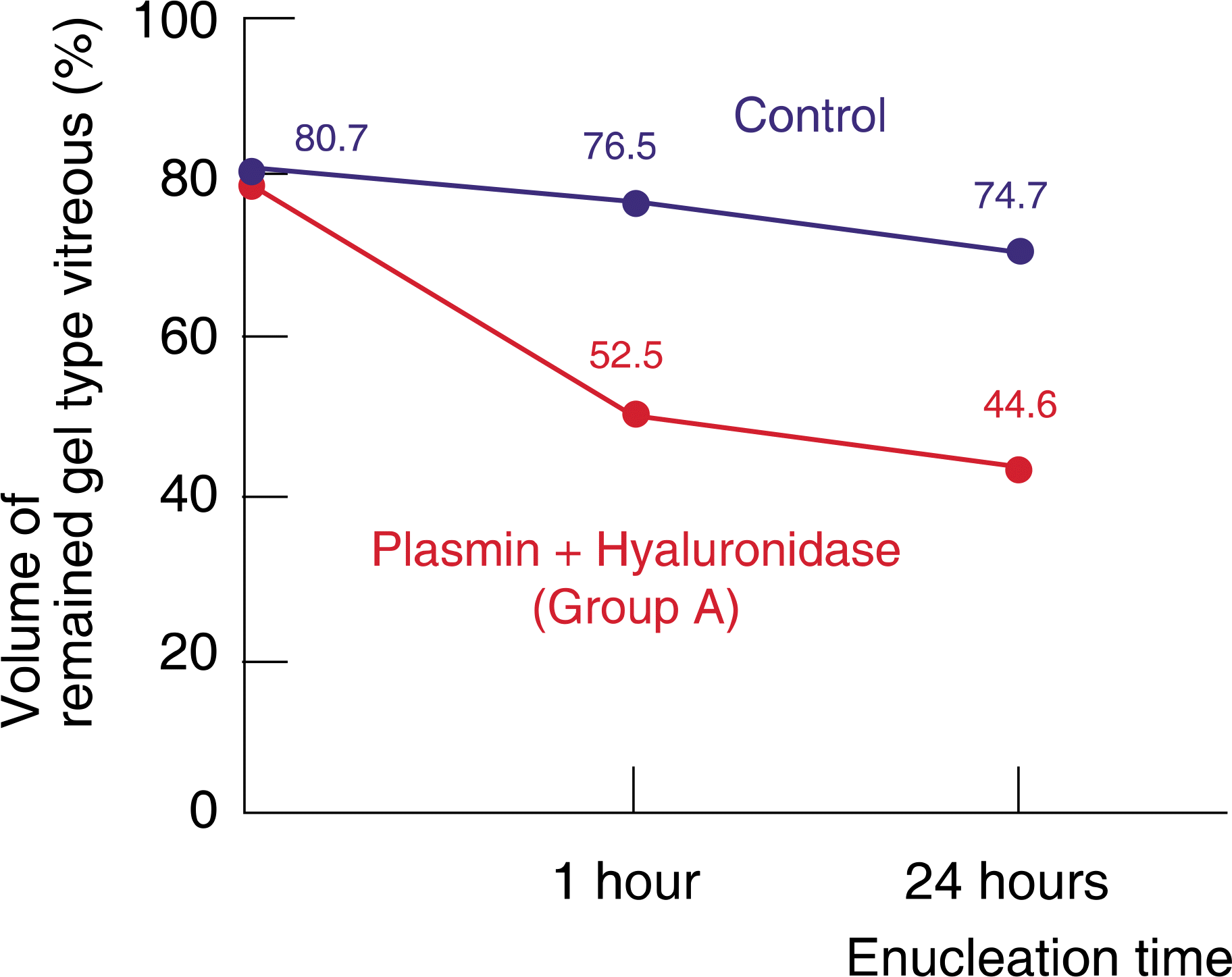 | Figure 2.Comparison of the plasmin and hyaluronidase injection group with the control group. At 1 hour after enucleation, volume of gel type vitreous per whole vitreous was 52.5% and 76.5% in group A and the control group, respectively. At 24 hours after enucleation, volume of gel type vitreous per whole vitreous was 44.6% and 74.7%, respectively. Volume of gel type vitreous per whole vitreous showed statistically significant difference between group A and the control group (1 hour: p=0.000; 24 hours: p=0.000). |
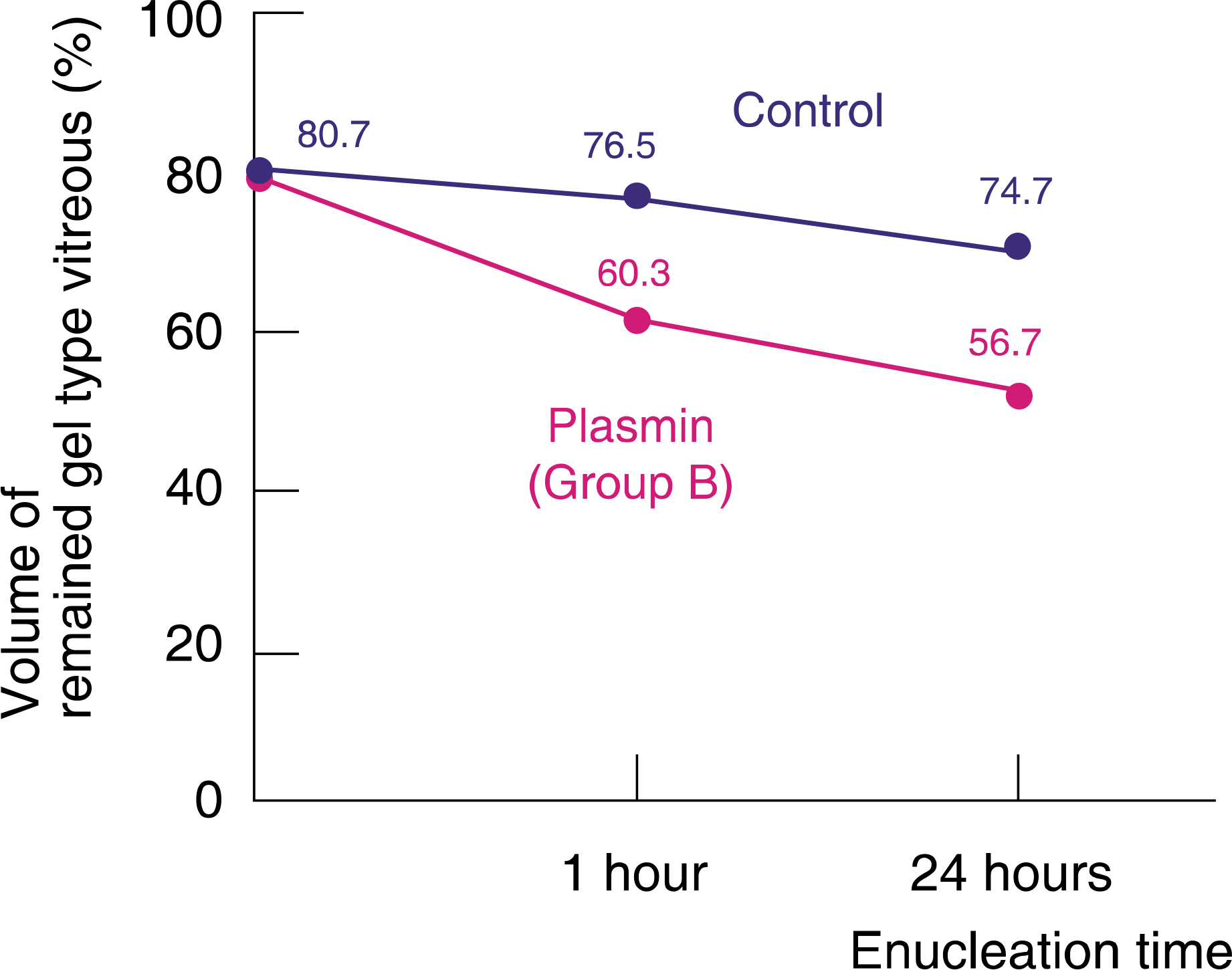 | Figure 3.Comparison of the plasmin injection group with the control group. At 1 hour after enucleation, volume of gel type vitreous per whole vitreous was 60.3% and 76.5% in group B and the control group, respectively. At 24 hours after enucleation, volume of gel type vitreous per whole vitreous was 56.7% and 74.7%, respectively. volume of gel type vitreous per hole vitreous showed statistically significant difference between group A and the control group (1 hour: p=0.000; 24 hours: p=0.000). |
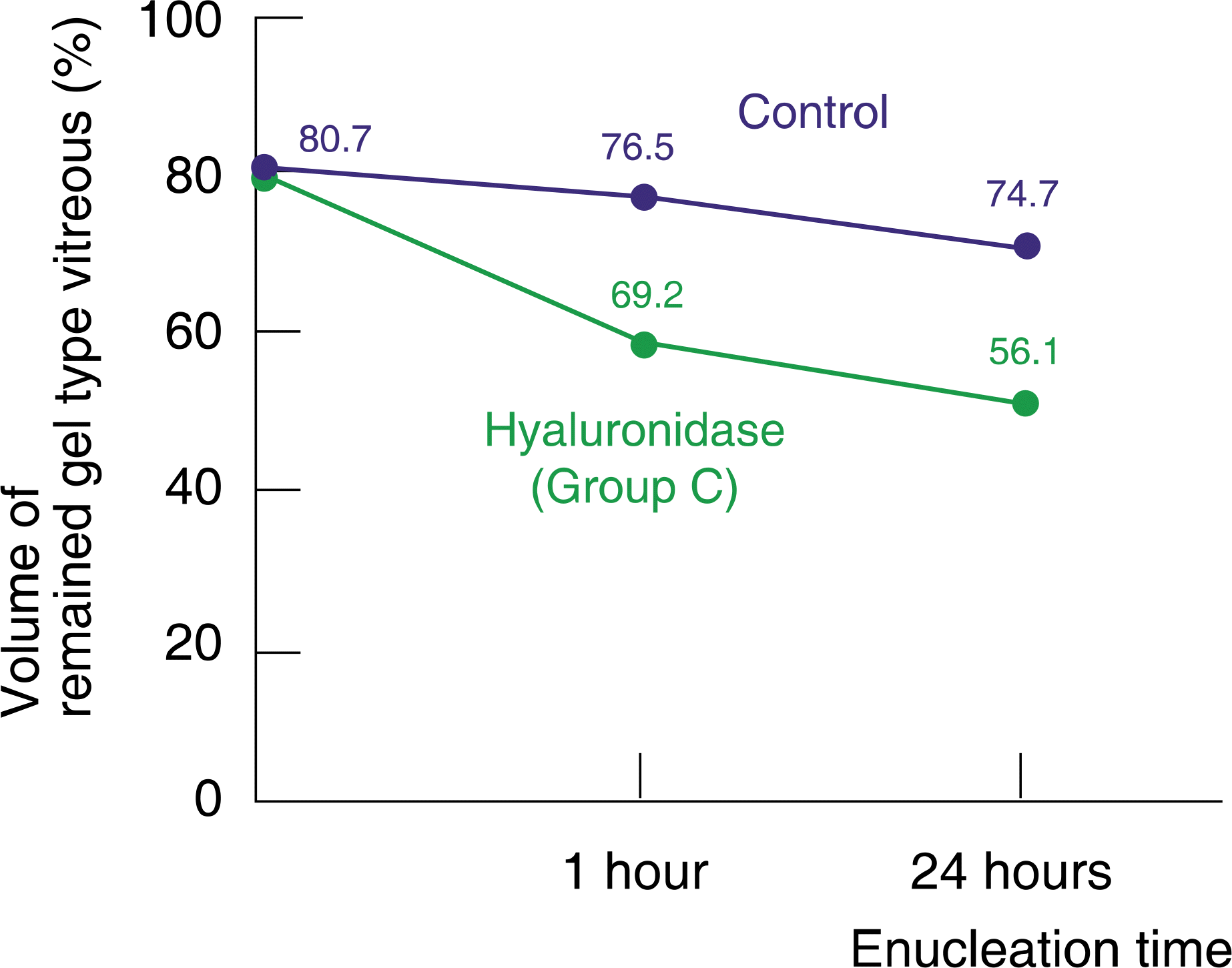 | Figure 4.Comparison of the hyaluronidase injection group with the control group. At 1 hour after enucleation, volume of gel type vitreous per whole vitreous was 69.2% and 76.5% in group C and the control group, respectively. At 24 hours after enucleation, volume of gel type vitreous per whole vitreous was 56.1% and 74.7%, respectively. volume of gel type vitreous per whole vitreous showed statistically significant difference between group A and the control group (1 hour: p=0.000; 24 hours: p=0.000). |
Table 1.
The mean volume of remained gel type vitreous
| |
Mean volume of gel type vitreous |
||
|---|---|---|---|
| I hour after enucleation | 24 hours after enucleation | p-valueП | |
| Group A* | 52.5±2.3% | 44.6±2.1% | p=0.018 |
| Group B† | 60.3±2.1% | 56.7±2.4% | p=0.000 |
| Group C‡ | 59.2±3.6% | 56.1±2.0% | p=0.179 |
| Control§ | 76.5±2.5% | 74.7±2.4% | |
Table 2.
Comparison of the mean volume of remained gel type vitreous between groups (at 24 hours after injection)
| | | Mean volume of gel type vitreous (%) | p value | |
|---|---|---|---|---|
| Group A* | Group B | 44.6±2.1 | 56.7±2.4 | 0.000 |
| Group C | 56.1±2.0 | 0.000 | ||
| Control§ | 74.7±2.4 | 0.000 | ||
| Group B† | Group C | 56.7±2.4 | 56.1±2.0 | 0.647 |
| Control | 74.7±2.4 | 0.000 | ||
| Group C‡ | Control | 56.1±2.0 | 74.7±2.4 | 0.000 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download