Abstract
Purpose
To evaluate the efficacy of the combination therapy of intravitreal bevacizumab injection and photodynamic therapy in neovascular age-related macular degeneration associated with large retinal pigment epithelial detachment.
Methods
A total of 13 eyes were reviewed, with 9 eyes diagnosed with definite choroidal neovascularization (CNV) and 4 eyes diagnosed with CNV or polypoidal choroidal vasculopathy (PCV) becausethe exact type could not be determined. Photodynamic therapy was performed within 1 week after bevacizumab injection according to indocyanine green angiography (ICGA). Additional bevacizumab injections were performed within a 4 to 6 week interval. Additional photodynamic therapy was performed within 4 months.
Results
The visual acuity on final examination had improved in 3 eyes (23.1%), was maintained in 7 eyes (53.8%), and decreased in 3 eyes (23.1%). The change of the PED before and after treatment showed regression in 5 eyes (38.5%), recurrence after regression in 2 eyes (15.4%), persistence in 4 eyes (30.8%), and retinal pigment epithelial tear in 2 eyes (15.4%). The maintained or improved visual acuity rate was 66.7% (6/9) and 100% (4/4) in the CNV and CNV or PCV group, respectively.
Conclusions
The combination therapy in neovascular age-related macular degenerationassociated with large retinal pigment epithelial detachment is a viable alternative treatment in the stabilization and improvement of vision. However, further studies with long-term follow up and controlled studies with anti-vascular endothelial growth factor antibody monotherapy are required.
References
1. Dastgheib K, Green WR. Granulomatous reaction to Bruch's membrane in age-related macular degeneration. Arch Ophthalmol. 1994; 112:813–8.

2. Oh H, Takagi H, Takagi C, et al. The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1999; 40:1891–8.
3. Ferrara N. Vascular endothelial growth factor. The trigger for neovascularization in the eye. Lab Invest. 1995; 72:615–18.
4. Adamis SP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005; 25:111–8.

5. Spaide RF, Sorenson J, Maranan L. Combined photodynamic therapy with verteporfin and intravitreal triamcinolone acetonide for choroidal neovascularization. Ophthalmology. 2003; 110:1517–25.

6. Chan WM, Lai TY, Wong AL, et al. Combined photodynamic therapy and intravitreal triamcinolone injection for the treatment of subfoveal choroidal neovascularization in age related macular degeneration: a comparative study. Br J Ophthalmol. 2006; 90:337–41.
7. Freund KB, Klais CM, Eandi CM, et al. Sequenced combined intravitreal triamcinolone and indocyanine green angiography-guided photodynamic therapy for retinal angiomatous proliferation. Arch Ophthalmol. 2006; 124:487–92.

8. Dhalla MS, Shah GK, Blinder KJ, et al. Combined photodynamic therapy with verteporfin and intravitreal bevacizumab for choroidal neovascularization in age-related macular degeneration. Retina. 2006; 26:988–93.

9. Costa RA, Jorge R, Calucci D, et al. Intravitreal bevacizumab (Avastin) in combination with verteporfin photodynamic therapy for choroidal neovascularization associated with age-related macular degeneration (IBeVe Study). Graefes Arch Clin Exp Ophthalmol. 2007; 245:1273–80.

10. Smith BT, Dhalla MS, Shah GK, et al. Intravitreal injection of bevacizumab combined with verteporfin photodynamic therapy for choroidal neovascularization in age-related macular degeneration. Retina. 2008; 28:675–81.

11. Ladewig MS, Karl SE, Hamelmann V, et al. Combined intravitreal bevacizumab and photodynamic therapy for neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2008; 246:17–25.

12. Krzystolik MG, Afshari MA, Adamis AP, et al. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol. 2002; 120:338–46.

13. Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an antivascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006; 26:859–70.

14. Schmidt-Erfurth U, Hasan T. Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age-related macular degeneration. Surv Ophthalmol. 2000; 45:195–214.

15. Schmidt-Erfurth U, Schlötzer-Schrehard U, Cursiefen C, et al. Influence of photodynamic therapy on expression of vascular endothelial growth factor (VEGF), VEGF receptor 3, and pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2003; 44:4473–80.

16. Spaide RF. Rationale for combination therapies for choroidal neovascularization. Am J Ophthalmol. 2006; 141:149–56.

17. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials—TAP report. Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Arch Ophthalmol. 1999; 117:1329–45.
18. Meredith TA, Braley RE, Aaberg TM. Natural history of serous detachments of the retinal pigment epithelium. Am J Ophthalmol. 1979; 88:643–51.

19. Poliner LS, Olk RJ, Burgess D, et al. Natural history of retinal pigment epithelial detachments in age-related macular degeneration. Ophthalmology. 1986; 93:543–50.

20. Elman MJ, Finde SL, Murphy RP, et al. The naturalhistory of serous retinal pigment epithelium detachment in patients with age-related macular degeneration. Ophthalmology. 1986; 93:224–30.
21. Pauleikhoff D, Löffert D, Spital G, et al. Pigment epithelial detachment in the elderly. Clinical differentiation, natural course and pathogenetic implications. Graefes Arch Clin Exp Ophthalmol. 2002; 240:533–8.
22. Slakter JS, Yannuzzi LA, Sorenson JA, et al. A pilot study of indocyanine green videoangiography guided laser photocoagulation treatment of occult choroidal neovascularization. Arch Ophthalmol. 1994; 112:465–72.
23. Lim JI, Aaberg TM, Capone A, Sternberg P. Indocyanine green angiography-guided photocoagulation of choroidal neovascularization associated with retinal pigment epithelial detachment. Am J Ophthalmol. 1997; 123:524–32.

24. Brancato R, Introini U, Bolognesi G, et al. ICGA-guided laser photocoagulation of occult choroidal neovascularization in age-related macular degeneration. Retina. 2000; 20:134–42.

25. Han JW, Lee WK. Photodynamic therapy of choroidal neovascularization associated with large serous pigment epithelial detachment. J Korean Ophthalmol Soc. 2004; 45:79–86.
26. Axer-Siegel R, Rosenblatt I, Kramer M, et al. Photodynamic therapy for occult choroidal neovascularization with pigment epithelium detachment in age-related macular degeneration. Arch Ophthalmol. 2004; 122:453–9.
27. Ladas ID, Kotsolis AI, Rouvas A, et al. Efficacy of photodynamic therapy in the management of occult choroidal neovascularization associated with serous pigment epithelium detachment. Ophthalmologica. 2007; 221:313–9.

28. Bom Aggio F, Eid Farah M, Melo GB. Intravitreal bevacizumab for occult choroidal neovascularization with pigment epithelium detachment in age-related macular degeneration. Acta Ophthalmol Scand. 2006; 84:713–4.

29. Frimpong-Boateng A, Varde MA, Rüfer F, et al. Intravitreal administration of triamcinolone and bevacizumab for pigment epithelial detachment in conjunction with AMD. Ophthalmologe. 2008; 105:661–8.
30. Chen E, Kaiser RS, Vander JF. Intravitreal bevacizumab for refractory pigment epithelial detachment with occult choroidal neovascularization in age-related macular degeneration. Retina. 2007; 27:445–50.

31. Ladas ID, Kotsolis AI, Papakostas TD, et al. Intravitreal bevacizumab combined with photodynamic therapy for the treatment of occult choroidal neovascularization associated with serous pigment epithelium detachment in age-related macular degeneration. Retina. 2007; 27:891–6.

32. Coscas G, Koenig F, Soubrane G. The pretear characteristics of pigment epithelial detachments. A study of 40 eyes. Arch Ophthalmol. 1990; 108:1687–93.
33. Chang LK, Sarraf D. Tears of the retinal pigment epithelium: an old problem in a new era. Retina. 2007; 27:523–34.
34. Chan CK, Meyer CH, Gross JG, et al. Retinal pigment epithelial tears after intravitreal bevacizumab injection for neovascular age-related macular degeneration. Retina. 2007; 27:541–51.

35. Casswell AG, Kohen D, Bird AC. Retinal pigment epithelial detachments in the elderly: classification and outcome. Br J Ophthalmol. 1985; 69:397–403.

36. Pece A, Isola V, Vadala M, Calori G. Photodynamic therapy with verteporfin for choroidal neovascularization associated with retinal pigment epithelial detachment in age-related macular degeneration. Retina. 2007; 27:342–8.

37. Meyer CH, Mennel S, Schmith JC, et al. Acute retinal pigment epithelial tear following intravitreal bevacizumab (Avastin) injection for occult choroidal neovascularization secondary to age related macular degeneration. Br J Ophthalmol. 2006; 90:1207–8.
38. Chang LK, Flaxel CJ, Lauer AK, Sarraf D. RPE tears after pegaptanib treatment in age-related macular degeneration. Retina. 2007; 27:857–63.

39. Goldstein M, Heilweil G, Barak A, et al. Retinal pigment epithelial tear following photodynamic therapy for choroidal neovascularization secondary to AMD. Eye. 2005; 9:1315–24.

40. Gass JD. Retinal pigment epithelial rip during krypton red laser photocoagulation. Am J Ophthalmol. 1984; 98:700–6.
Figure 1.
Indocyanine green angiography (ICGA) of case 11 (A, B) and case 13 (C, D) at the initial visit (A, C) and before combined treatment of intravitreal bevacizumab injection and photodynamic therapy (PDT) (B, D). At the initial visit of case 11, ICGA demonstrates neovascular nets with pigment epithelial detachment (PED) (A). After second treatment of PDT, persisted neovascular net mixed with newly appearing polypoidal like lesion on ICGA (B). At initial visit of case 13, ICGA demonstrates a cluster of polypoidal lesion with large PED (C). After singlecombined treatment of intravitreal triamcinolone injection and PDT, noted the regression of cluster of polypoidal lesion with PED, and then recurred the lesion changed typical neovascular nets with PED on ICGA (D).

Figure 2.
Distribution of final visual outcome for patients having previous treatment and no previous treatment; VA=visual acuity.
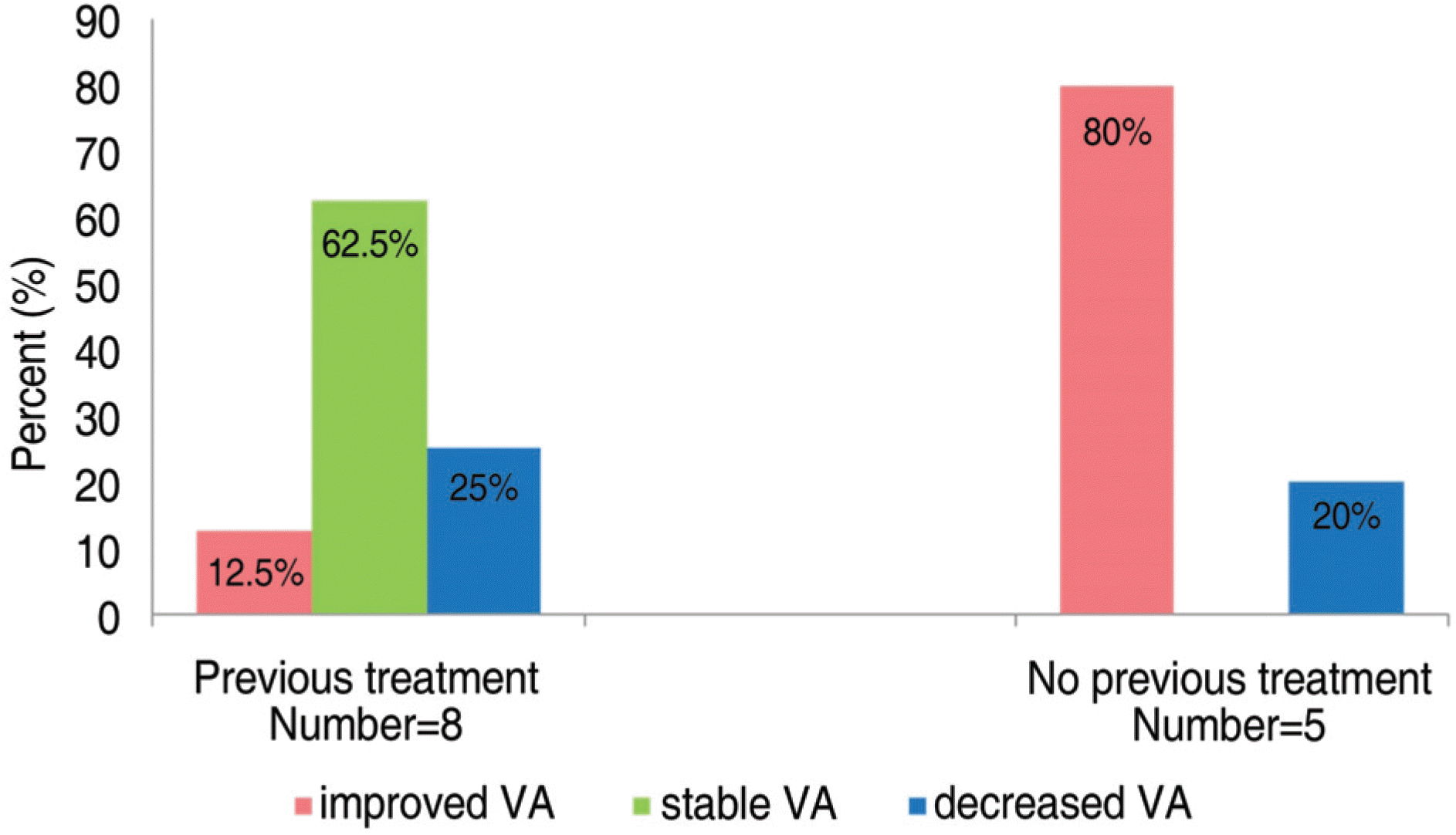
Figure 3.
Fundus photographs, fluorescein angiography (FA), optical coherence tomography (OCT) and indocyanine green angiography (ICGA) of case 2 at baseline (A-C) and at 3 months after single combined treatment and consecutive two additional bevacizumab injection (D-F). Note the resolution of the choroidal neovascularization and pigment epithelial detachment on FA, OCT and ICGA.

Figure 4.
Fundus photographs, fluorescein angiography (FA) and indocyanine green angiography (ICGA) of case 6 at baseline (A-C), at 3 months after first combined treatment (D-F) and at 3 months after second combined treatment and one additional bevacizumab injection (G-I). Note the persistent of pigment epithelial detachment and choroidal neovascularization on FA and ICGA, but visual acuity remained stable.

Figure 5.
Fundus photographs, fluorescein angiography (FA), optical coherence tomography (OCT) and indocyanine green angiography (ICGA) of case 8. At baseline, showing choroidal neovascularization with pigment epithelial detachment (PED) (A-B). At 2 months after single combined treatment and consecutive two additional bevacizumab injection, showing the retinal pigment epithelial (RPE) tear on fundus photograph, FA, OCT, and ICGA (C-E). The arrowhead on OCT demonstrates RPE rip at the edge of detached RPE (C), but visual acuity was decreased within the 2 lines. At the 15 months, showing the subretinal fluid on OCT and marked decrease of visual acuity (F).

Figure 6.
Distribution of final visual outcome of CNV group and CNV or PCV group; CNV=choroidal neovascularization; PCV=polypoidal choroidal vasculopathy; VA= visual acuity.
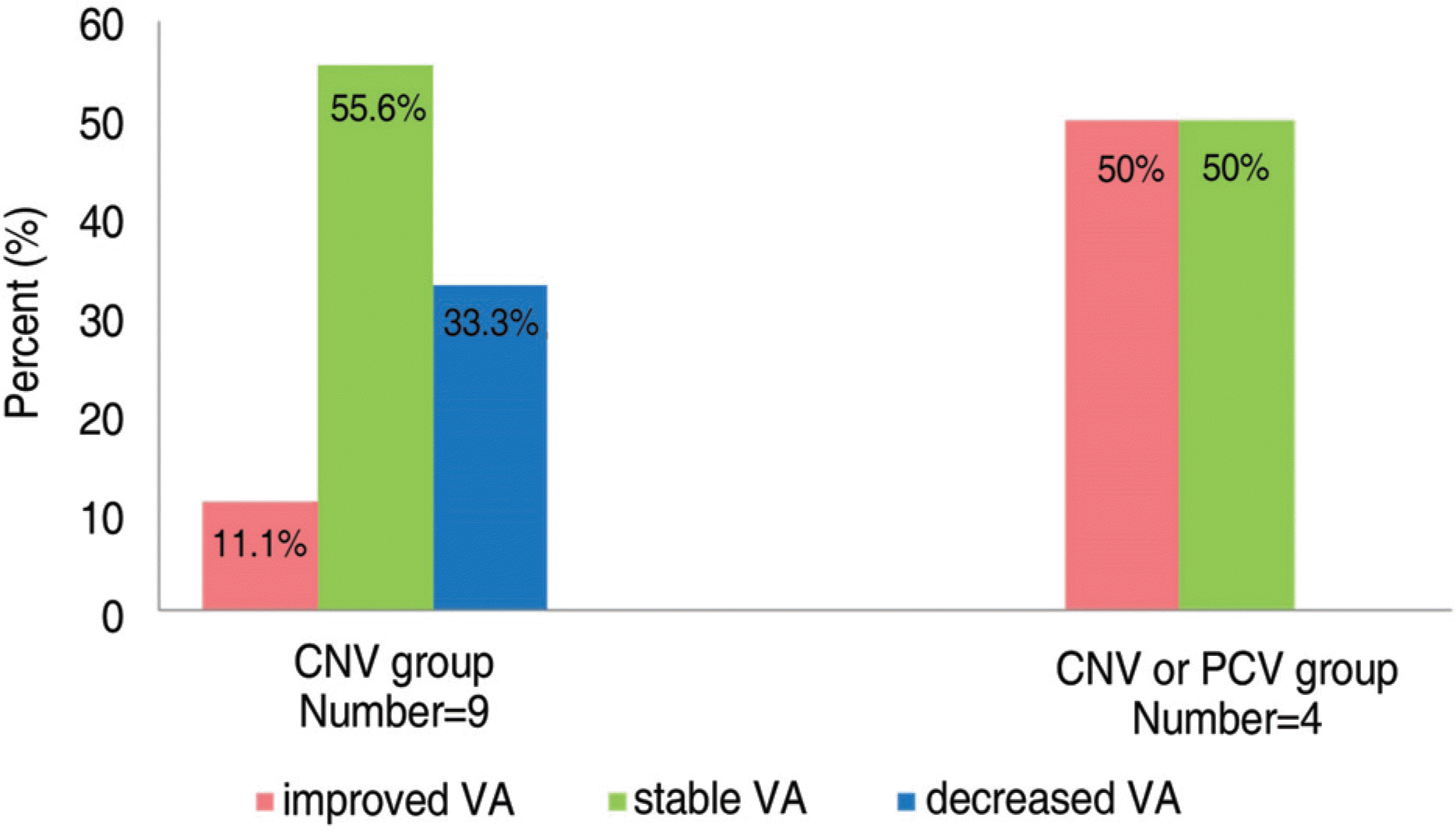
Table 1.
Demographic data of patients
| Pt. | Dx. | Age/ Sex | PED* Size (DD†) | Previous treatment | Combination treatment | F/U (m‡) | logMAR BCVA§ | PED | Abnormal vessel | |
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Final | |||||||||
| 1 | CNV∏ | 64/F | 2.53 | no | Combo** 1(add avastin 2) | 10 | 0.3 | 0.1 | regression | regression |
| 2 | CNV | 73/F | 1.87 | no | Combo 1(add avastin 2) | 11 | 0.2 | 0.1 | regression | regression |
| 3 | CNV | 72/F | 1.87 | PDT 2+IVTA 2 | Combo 1(add avastin 1) | 14 | 0.4 | 0.4 | recur | recur |
| 4 | CNV | 76/M | 2.33 | PDT 1+IVTA 1 | Combo 3(add avastin 2) | 15 | 0.3 | 0.6 | recur | recur |
| 5 | CNV | 67/M | 2.03 | PDT 1, IVTA 1 | Combo 1 | 8 | 0.3 | 0.3 | persist | persist |
| 6 | CNV | 66/M | 3.70 | Laser 1, IVTA 1, PDT 1 | Combo 2(add avastin 1) | 10 | 0.5 | 0.5 | persist | persist |
| 7 | CNV | 62/M | 2.60 | PDT 3+IVTA 3 | Combo 2(add avastin 2) | 12 | 0.2 | 0.2 | persist | persist |
| 8 | CNV | 79/M | 2.43 | No | Combo 1(add avastin 2) | 16 | 0.2 | 1.4 | RPE tear | persist |
| 9 | CNV | 79/F | 2.72 | PDT 1+IVTA 1 | Combo 1 | 13 | 0.7 | 1.1 | RPE tear | regression |
| 10 | CNV or PCV# | 77/M | 1.18 | no | Combo 1 | 10 | 1.4 | 1.1 | regression | regression |
| 11 | CNV or PCV | 75/M | 1.83 | PDT 2 | Combo 1(add avastin 1) | 9 | 0.7 | 0.2 | regression | regression |
| 12 | CNV or PCV | 77/M | 1.98 | No | Combo 1(add avastin 1) | 11 | 1.2 | 1.1 | regression | regression |
| 13 | CNV or PCV | 67/M | 2.5 | IVTA 1+PDT 1 | Combo 1(add avastin 2) | 7 | 0.2 | 0.2 | persist | persist |
Table 2.
Subdivide into final result of CNV and PCV or CNV group according to serial state of PED and abnorma vessel and visual outcome
| | PED* | | Abnormal vessel | | Visual outcome |
|---|---|---|---|---|---|
| CNV† group (n:9) | regression (n:2) | → | regression (n:2) | → | improve (n:1) |
| | | | | | stable (n:1) |
| | persist (n:3) | → | persist (n:3) | → | stable (n:3) |
| | recur (n:2) | → | recur (n:2) | → | stable (n:1) |
| | | | | | decrease (n:1) |
| | RPE§ tear (n:2) | → | regression (n:1) | → | decrease (n:2) |
| | | | persist (n:1) | | |
| CNV or PCV‡ | regression (n:3) | → | regression (n:3) | → | improve (n:2) |
| group (n:4) | | | | | stable (n:1) |
| | persist (n:1) | → | persist (n:1) | → | stable (n:1) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download