Abstract
Purpose
To evaluate the efficacy of a prophylactic posterior sub-Tenon's capsule injection of Triamcinolone acetonide (TA) against macular edema and visual dysfunction by panretinal photocoagulation (PRP) in patients with severe nonproliferative and proliferative diabetic retinopathy.
Methods
Thirty-eight eyes of 19 patients who have diabetic retinopathy without macular edema and whose retinopathy was bilateral and symmetrical were evaluated. Triamcinolone was injected into the posterior sub-Tenon's capsule in one eye of the patients and nothing was injected in the other eye as a control. Two weeks later, PRP was performed every other week for 4 sessions on both eyes in all patients. The clinical course of visual acuity and macular edema was monitored for up to approximately 6 months after the initial PRP.
Results
There was no statistically significant difference of visual acuity before PRP in the 2 groups (p>0.05), and there was no macular edema in any patient. For a follow-up period of 6 months, visual dysfunction was more severe in the TA-injected eye than the control. However, the difference was not statistically significant (p>0.05) throughout the follow-up period except at the 20-week time point. On the other hand, macular edema occurred in 2 eyes (10.5%) of the TA-injected group, and in 4 eyes (21.1%) of the control group. IOP elevation as a complication caused by TA-injection occurred in 2 eyes (10.5%). However, IOP was controlled successfully by anti-glaucomatic eye drops.
Go to : 
References
1. Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy, ETDRS report number 9. Ophthalmology. 1991; 98:766–85.
2. Early Treatment Diabetic Retinopathy Study Research Group. Early treatment diabetic retinopathy study design and baseline patient characteristics, ETDRS report number 7. Ophthalmology. 1991; 98:741–56.
3. Diabetic Retinopathy Study Research Group. Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol. 1976; 81:383–96.
4. The Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical applications of DRS findings. DRS Report No 8. Ophthalmology. 1981; 88:583–600.
5. Verma LK, Vivek MB, Kumar A, et al. A prospective controlled trial to evaluate the adjunctive role of posterior subtenon triamcinolone in the treatment of diffuse diabetic macular edema. J Ocular Pharmacol Ther. 2004; 20:277–84.

6. Meyer-Schwickerath GR, Schott K. Diabetic retinopathy and photocoagulation. Am J Ophthalmol. 1968; 66:597–603.

7. Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002; 109:920–7.

8. Tano Y, Chandler D, Machemer R. Treatment of intraocular proliferation with intravitreal injection of triamcinolone acetonide. Am J Ophthalmol. 1980; 90:810–6.

9. Ozdek S, Bahceci UA, Gurelik G, Hasanreisoglu B. Posterior subtenon and intravitreal triamcinolone acetonide for diabetic macular edema. J Diabetes Complications. 2006; 20:246–51.
10. Bakri SJ, Kaiser PK. Posterior subtenon triamcinolone for refractory diabetic macular edema. Am J Ophthalmol. 2005; 139:290–4.
11. Bonini-Filbo MA, Jorge R, Barbosa JC, et al. Intravitreal injection versus subtenon's infusion of triamcinolone acetonide for refractory diabetic macular edema: a randomized clinical trial. Ophthalmol Vis Sci. 2005; 46:3845–9.
12. Cardillo JA, Melo LA, Cost RA, et al. Comparison of intravitreal versus posterior subtenon's capsule injection of triamcinolone acetonide for diffuse diabetic macular edema. Ophthalmology. 2005; 112:1557–63.

13. Lee YH, Kim CG. Intravitreal triamcinolone acetonide injection for treatment of macular edema. J Korean Ophthalmol Soc. 2004; 45:2055–63.
14. Zacks DN, Johnson MW. Combined intravitreal injection of triamcinolone acetonide and panretinal photocoagulation for concomitant diabetic macular edema and proliferative diabetic retinopathy. Retina. 2005; 25:135–40.

15. Henricsson M, Heijl A. The effect of panretinal laser photocoagulation on visual acuity, visual fields and on subjective visual impairment in preproliferative and early proliferative diabetic retinopathy. Acta Ophthalmol. 1994; 72:570–5.

16. Gentile RC, Stegman Z, Liebmann JM, et al. Risk factors for choroidal effusion after panretinal photocoagulation. Ophthalmology. 1996; 103:827–32.
17. Lam DS, Chan CK, Tang EW, et al. Intravitreal triamcinolone for diabetic macular edema in Chinese patients: six-month prospective longitudinal pilot study. Clin Experiment Ophthalmol. 2004; 32:569–72.
18. Islam MS, Vemon SA, Negi A. Intravitreal triamcinolone will cause posterior subcapsular cataract in most eyes with diabetic maculopathy within 2 years. Eye. 2006; 17:1–3.

19. Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003; 136:791–6.

20. Nelson ML, Tennant MT, Sivalingam A, et al. Infectious and presumed noninfectious endophthalmitis after intravitreal triamcinolone acetonide injection. Retina. 2003; 23:686–91.

21. Roth DB, Chieh J, Spirn MJ, et al. Noninfectious endophthalmitis associated with intravitreal triamcinolone injection. Arch Ophthalmol. 2003; 121:1279–82.

22. Jonas JB, Kreissig I, Degenring RF. Endophthalmitis after intravitreal injection of triamcinolone injections. Arch Ophthalmol. 2003; 121:1279–82.
23. Craig JH, Gray NH. The effects of posterior subtenon injection of triamcinolone acetonide in patients with intermediate uveitis. Am J Ophthalmol. 1995; 120:55–64.
Go to : 
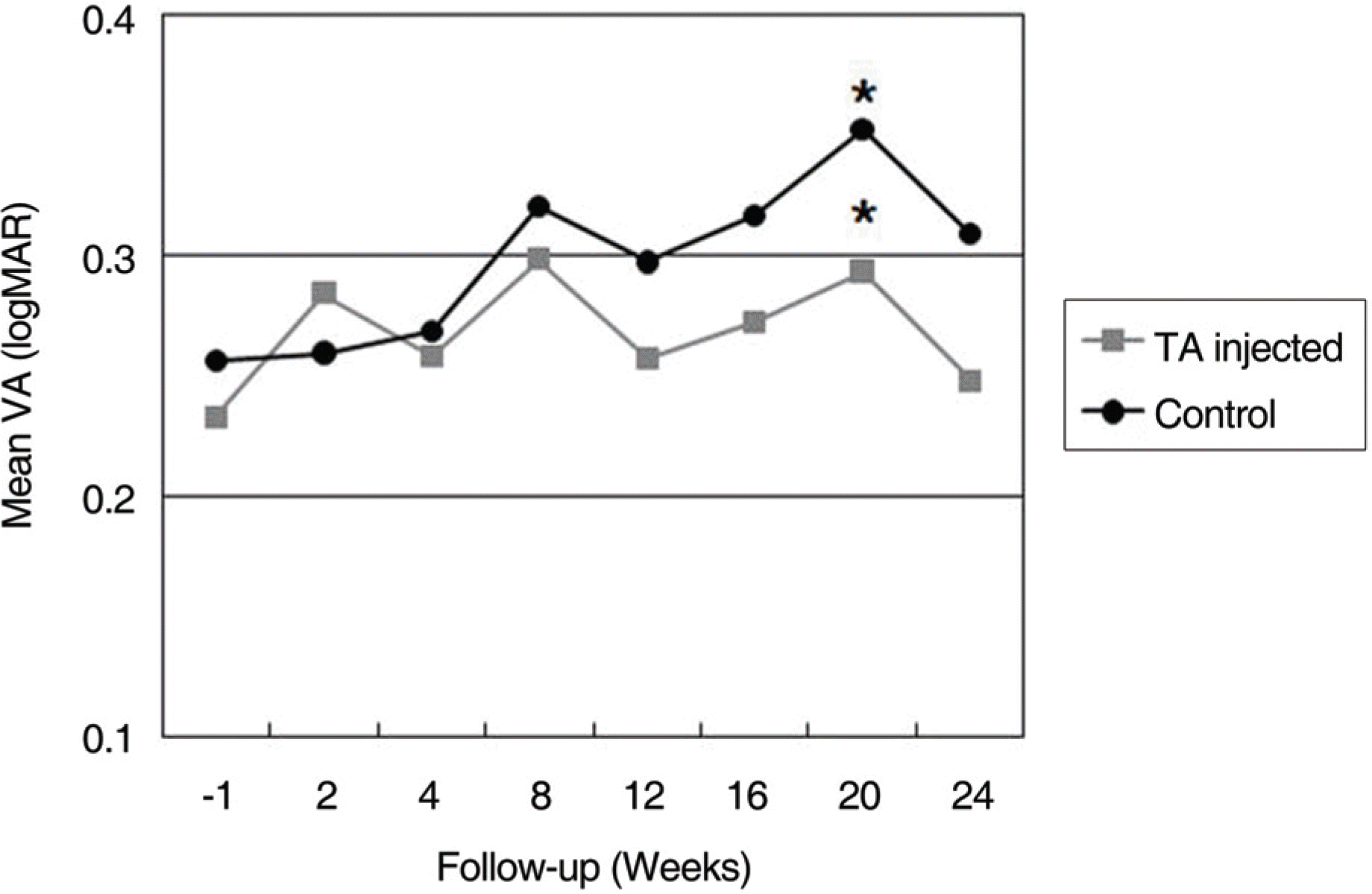 | Figure 1.Line graph illustrating of the clinical course of logarithm of the minimum angle of resolution (logMAR) visual acuity between the TA-inejcted and control eyes. There was not statistically significant difference between two groups before TA injection and after 2 weeks. TA-injected group shows relatively good vision than control group through the follow-up period. But the difference was not statistically significant (p>0.05) except at 20 weeks follow-up time point (p<0.05). (* statistically significant difference between the TA-injected and control eyes at the point) |
Table 1.
Baseline clinical characteristics of 19 patients
| Patients No. | Age (yrs) | Sex | DM* duration (yrs) | HbA1c (%) | Hypertension | Lens |
|---|---|---|---|---|---|---|
| 1 | 47 | M‡ | 1.5 | 9.8 | − | P∏ |
| 2 | 58 | F† | 6 | 7.9 | − | P |
| 3 | 47 | F | 3 | 6.9 | + | P |
| 4 | 68 | M | 5 | | − | P |
| 5 | 60 | F | 15 | 12.1 | − | P |
| 6 | 63 | F | 14 | 11.2 | − | IOL‡ |
| 7 | 65 | F | 19 | 7.3 | + | IOL |
| 8 | 62 | M | 28 | 6.8 | − | P |
| 9 | 49 | F | 11 | | − | P |
| 10 | 67 | F | 1 | 8 | − | P |
| 11 | 54 | F | 11 | 13 | − | P |
| 12 | 57 | F | 12 | | − | P |
| 13 | 52 | M | 6 | 13.8 | − | P |
| 14 | 67 | F | 23 | 8.3 | − | IOL |
| 15 | 64 | F | 3 | 6.9 | + | P |
| 16 | 53 | M | 11 | 6.1 | − | P |
| 17 | 48 | M | 2 | 6.6 | − | P |
| 18 | 66 | F | 12 | 10.9 | − | P |
| 19 | 65 | F | 20 | | + | P |
| Mean | 58.53 | | 10.71 | 9.04 | | |
| SD** | 7.46 | | 7.78 | 2.55 | | |
| Maximum | 68 | | 28 | 13.8 | | |
| Minimum | 47 | | 1 | 6.1 | | |
Table 2.
Alteration of visual acuity (logMAR*) after TA treatment
| Patient No. |
Triamcinolone Acetonide Injected |
Control |
||||
|---|---|---|---|---|---|---|
| −1 week | 2 weeks | 24 weeks | −1 week | 2 weeks | 24 weeks | |
| 1 | 0.000 | 0.222 | 0.097 | 0.097 | 0.097 | 0.097 |
| 2 | 0.301 | 0.222 | 0.301 | 0.222 | 0.301 | 0.222 |
| 3 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.301 |
| 4 | 0.046 | 0.097 | 0.046 | 0.097 | 0.046 | 0.155 |
| 5 | 0.155 | 0.097 | 0.097 | 0.046 | 0.097 | 0.398 |
| 6 | 0.155 | 0.155 | 0.222 | 0.155 | 0.222 | 0.222 |
| 7 | 0.097 | 0.155 | 0.097 | 0.155 | 0.097 | 0.155 |
| 8 | 0.097 | 0.523 | 0.155 | 0.000 | 0.155 | 0.222 |
| 9 | 0.699 | 0.523 | 1.000 | 0.523 | 1.000 | 0.523 |
| 10 | 0.301 | 0.523 | 0.398 | 0.699 | 0.398 | § |
| 11 | 0.155 | 0.222 | 0.155 | 0.155 | 0.155 | 0.301 |
| 12 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 13 | 1.222 | 1.398 | 1.222 | 1.398 | 1.222 | 1.699 |
| 14 | 0.222 | ‡ | 0.046 | 0.155 | ‡ | 0.046 |
| 15 | 0.222 | 0.222 | 0.222 | 0.222 | 0.222 | 0.222 |
| 16 | 0.222 | 0.398 | 0.301 | 0.398 | 0.301 | 0.155 |
| 17 | 0.222 | 0.097 | 0.097 | 0.222 | 0.097 | 0.398 |
| 18 | 0.155 | 0.097 | 0.097 | 0.097 | 0.097 | 0.222 |
| 19 | 0.155 | 0.155 | 0.155 | 0.222 | 0.155 | 0.222 |
| Mean | 0.233 | 0.284 | 0.248 | 0.256 | 0.259 | 0.309 |
| SD† | 0.286 | 0.325 | 0.324 | 0.329 | 0.329 | 0.369 |
| Maximum | 1.222 | 1.398 | 1.000 | 1.398 | 1.222 | 1.699 |
| Minimum | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.046 |
Table 3.
Results of treatment and complication




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download