Abstract
Purpose
We evaluated the causative microorganisms, dispositions, and visual prognosis of endogenous endophthalmitis.
Methods
The records of 12 eyes of 10 patients who were diagnosed with endogenous endophthalmitis were retrospectively reviewed with respect to the causative microorganisms, dispositions, visual prognosis, initial visual acuity, time interval between onset of symptom, and the course of treatment.
Results
Three of the four eyes infected with a candida species recovered a visual acuity of 0.1 or better; none of the other infected eyes reached higher than 0.1 (p=0.024). Initial visual acuity of less than hand movement reached globe loss in three eyes, while 5 eyes with initial visual acuity of better than hand movement of seven eyes did not lose the globe (p=0.045). Three out of patients with a time interval between onset of symptoms and treatment of more than five days did not lose the globe, while those with a time interval of less than five days of eight eyes did not lose the globe.
Conclusions
Visual prognosis and globe survival depends mainly on the underlying microorganism, initial visual acuity, the time interval between the onset of symptom, and treatment. Patients with candida endophthalmitis had good visual outcome compared with the others, and thus microbial culture identification is a useful predictor of visual acuity and treatment.
Go to : 
References
1. Hassan IJ, MacGowan AP, Cook SD. Endophthalmitis at the Bristol Eye Hospital: an 11-year review of 47 patients. J Hosp Infect. 1992; 22:271–8.

2. Irvine WD, Flynn HW Jr, Miller D, et al. Endophthalmitis caused by gram negative organisms. Arch Ophthalmol. 1992; 110:1450–4.
3. Shrader SK, Band JD, Lauter CB, et al. The clinical spectrum of endophthalmitis: incidence, predisposing factors, and features influencing outcome. J Infect Dis. 1990; 162:115–20.

4. Stonecipher KG, Ainbinder DJ, Maxwell DP, et al. Infectious endophthalmitis: a review of 100 cases. Ann Ophthalmol. 1994; 26:108–15.
5. Bohigian GM, Olk RJ. Factors associated with a poor visual result in endophthalmitis. Am J Ophthalmol. 1986; 101:332–41.

6. Ness T, Pelz K, Hansen LL. Endogenous endophthalmitis: microorganisms, disposition and prognosis. Acta Ophthalmol Scand. 2007; 85:852–6.

7. Okada AA, Johnson RP, Liles WC, et al. Endogenous bacterial endophthalmitis: report of a ten-year retrospective study. Ophthalmology. 1994; 101:832–8.
8. Jackson TL, Eykyn SJ, Graham EM, Stanford MR. Endogenous bacterial endophthalmitis: a 17-year prospective series and review of 267 reported cases. Surv Ophthalmol. 2003; 48:403–23.

9. Wong JS, Chan TK, Lee HM, Chee SP. Endogenous Bacterial endophthalmitis: an east Asian experience and a reappraisal of a severe ocular affliction. Ophthalmology. 2000; 107:1483–91.

10. Chowdhury T, Jalali S, Majji AB. Successful treatment of fungal retinitis and retinal vasculitis with oral itraconazole. Retina. 2002; 22:800–2.

11. Biswas J. Fungal endophthalmitis after a single intravenous administration of presumably contaminated dextrose infusion fluid. Retina. 2001; 21:93–4.

12. Zhang YQ, Wang WJ. Treatment outcomes after pars plana vitrectomy for endogenous endophthalmitis. Retina. 2005; 25:746–50.

13. Binder MI, Chua J, Kaiser PK, et al. Endogenous endophthalmitis: an 18-year review of culture-positive cases at a tertiary care center. Medicine (Baltimore). 2003; 82:97–105.
14. Schiedler V, Scott IU, Flynn HW Jr, et al. Culture-proven endogenous endophthalmitis: clinical features and visual acuity outcomes. Am J Ophthalmol. 2004; 137:725–31.

15. Callegan MC, Engelbert M, Parke DW 2nd, et al. Bacterial endophthalmitis: epidemiology, therapeutics, and bacterium-host interactions. Clin Microbiol Rev. 2002; 15:111–24.

16. Oh TS, Ahn Y, Chang SD, Lee YK. A case of endogenous endophthalmitis caused By Klebsiella pneumoniae from emphysem-atous pyelonephritis. J Korean Ophthalmol Soc. 2002; 43:1330–4.
17. Lee SM, Lee JH. A case of Enterococcus faecalis endophthalmitis with corneal ulcer. J Korean Ophthalmol Soc. 2004; 18:175–9.

18. Bae JH, Lee SS. A case of Enterococcus faecalis endophthalmitis following ECCE. J Korean Ophthalmol Soc. 1994; 35:70–3.
19. Kim US, Yu SY, Kwak HW. Two cases of Enterococcus fecalis endophthalmitis. J Korean Ophthalmol Soc. 2003; 44:523–8.
20. Lee YH, Choi SJ, Kim IC, Chung YT. A case of the bilateral metastatic endophthalmitis. J Korean Ophthalmol Soc. 1995; 36:2048–53.
21. Byun YC, Lee H, Lee EK, Lee KW. A case of metastatic eno-phthalmitis originated from bacterial endocarditis. J Korean Ophthalmol Soc. 1994; 35:122–7.
22. Shim HS, Lee SS, Park JM, Song JK. Three cases of the metastatic endophthalmitis. J Korean Ophthalmol Soc. 1994; 35:350–5.
23. Chou FF, Kou HK. Endogenous endophthalmitis associated with pyogenic hepatic abscess. J Am Coll Surg. 1996; 182:33–6.
24. Cahill M, Chang B, Murray A. Bilateral endogenous bacterial endophthalmitis associated with pyogenic hepatic abscess. Br J Ophthalmol. 2000; 84:1436.

25. La TU, Kim CW, Lee JS. A case of endogenous endophthalmitis accompanying orbital cellulitis caused by Klebsiella pneumoniae from liver abscess. J Korean Ophthalmol Soc. 2000; 41:1000–5.
Go to : 
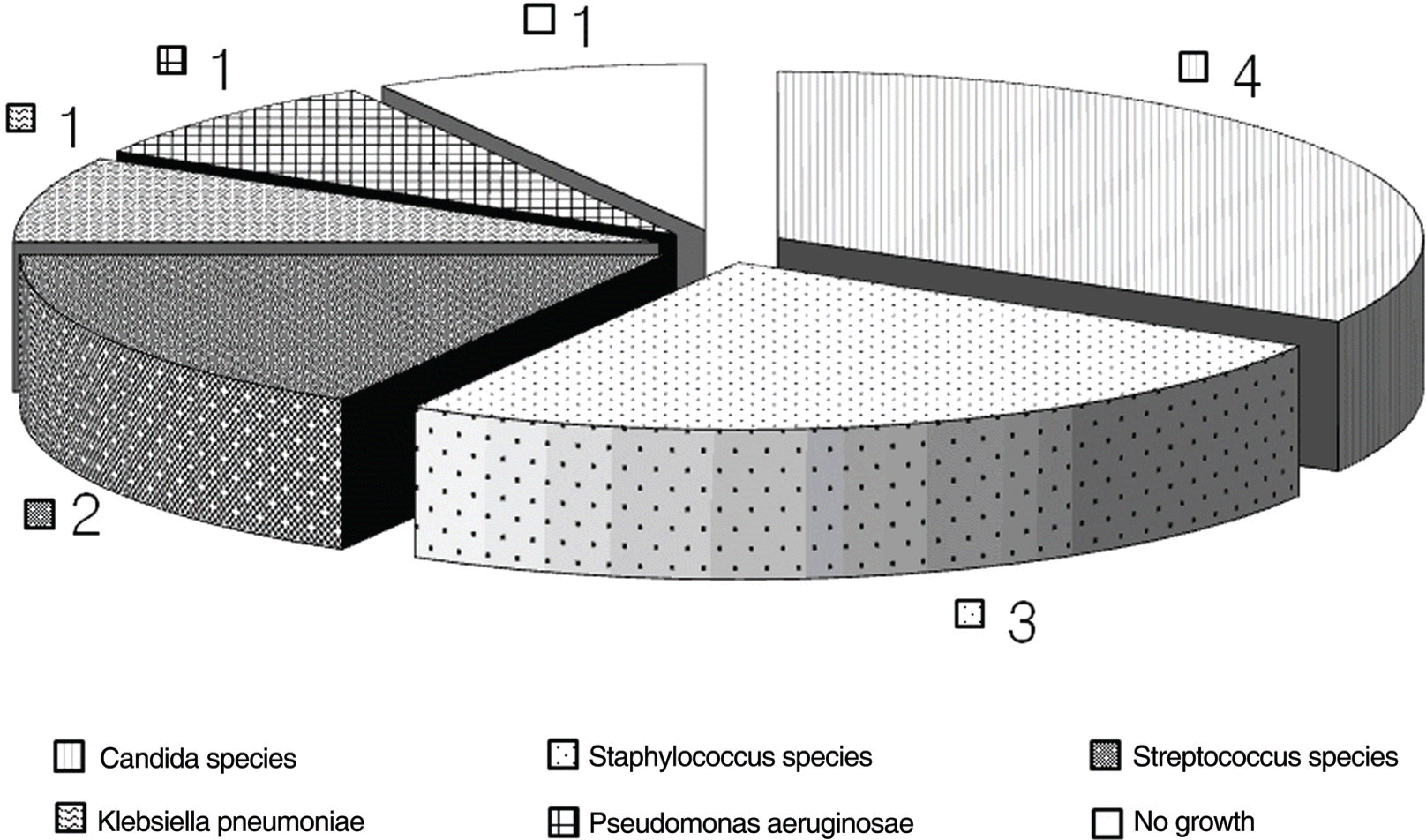
Table 1.
Clinical summary
| Case | Eye | Age/G | Organism | Risk factor | Initial V/A | Culture | Time (day) | IVT agent V | itrectomy | Final V/s |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | OD | 51/M | S. aureus | DM | CF | V* | 4 | Va‡, Ak§ | N | CF |
| | OS | 51/M | S. aureus | DM | 0.2 | V* | 4 | Va‡, Ak§ | N | 0.02 |
| 2 | OS | 91/F | None | Laryngeal ca′ | LP | None | 7 | Va‡, Ak§ | N | Evi# |
| 3 | OS | 62/F | S. pneumoniae | None | LP | V* | 10 | Va‡, Ak§ | N | Evi# |
| 4 | OS | 95/F | P. aeruginosa | None | NLP | V* | 8 | Va‡, Ak§ | N | Evi# |
| 5 | OS | 45/M | K. pneumoniae | DM, Liver cirrhosis, pneumoniae | 0.08 | B† | 4 | Va‡, CΠ, Amp | N | LP |
| 6 | OD | 69/M | C. albicans | Multiple myeloma | CF | V* | 7 | Va‡, Ak§, Amp | Y | 0.08 |
| 7 | OD | 39/M | C. albicans | Abdominal surgery | 0.3 | V*, B† | 3 | Va‡, Ak§, Amp | Y | 0.6 |
| 8 | OS | 47/M | S. aureus | DM | HM | V* | 3 | Va‡, Ak§ | Y | HM |
| 9 | OS | 46/M | S. viridans | DM | HM | V* | 4 | Va‡, Ak§ | Y | HM |
| 10 | OD | 58/M | C. albicans | DM, Liver abscess | 0.04 | V*, B† | 2 | Va‡, Ak§, Amp | Y | 0.4 |
| | OS | 58/M | C. albicans | DM, Liver abscess | 0.15 | V*, B† | 2 | Va‡, CΠ, Amp | Y | 0.1 |
Table 2.
Predisposing medical conditions
| Predisposing medical condition | N* |
|---|---|
| Diabetes | 7 |
| Liver abscess | 3 |
| Cancer | 1 |
| Hematologic malignancies | 1 |
| Recent severe surgical procedure | 1 |
| Pneumonia | 1 |
| None | 2 |
Table 3.
Outcome in eyes infected with candida and those infected with other microorganisms
| Microorganism | Globe loss | ≤0.02 | ≥0.1 |
|---|---|---|---|
| Candida species | 0/4 | 0/4 | 3/4 |
| Others | 2/7 | 5/7 | 0/7 |
| Staphylococcus species | 0/3 | 3/3 | 0/3 |
| Streptococcus species | 1/2 | 1/2 | 0/2 |
| Pseudomonas aeruginosa | 1/1 | 0/1 | 0/1 |
| Klebsiella pneumoniae | 0/1 | 1/1 | 0/1 |
| p-value* | 0.491 | 0.061 | 0.024† |
Table 4.
Outcome in eyes with initial V/A
| Initial V/A | Globe loss | ≤0.02 | ≥0.1 |
|---|---|---|---|
| ≤ HM | 3/5 | 2/5 | 0/5 |
| > HM | 0/7 | 3/7 | 3/7 |
| p-value* | 0.045† | 1.0 | 0.2 |
Table 5.
Outcome in eyes with time*
| Times* | Globe loss | ≤0.02 | ≥0.1 |
|---|---|---|---|
| ≤5 days | 0/8 | 5/8 | 3/8 |
| >5 days | 3/4 | 0/4 | 0/4 |
| p-value† | 0.017‡ | 0.082 | 0.4 |
Table 6.
Outcome in vitrectomy eyes and non-vitrectomy eyes
| Operation | Globe loss | ≤0.02 | ≥0.1 |
|---|---|---|---|
| Vitrectomy eyes | 0/6 | 2/6 | 3/6 |
| Non-vitrectomy eyes | 3/6 | 3/6 | 0/6 |
| p-value* | 0.18 | 1.0 | 0.18 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download