Abstract
Purpose
The effect of preoperative intravitreal bevacizumab (Avastin®) injection was investigated in primary vitrectomy for severe proliferative diabetic retinopathy.
Methods
Eyes that underwent vitectomy for proliferative diabetic retinopathy were followed up at least 6 months and were reviewed retrospectively. The authors reviewed functional outcomes, complications, and operation time between preoperative bevacizumab injection (group I) and non-injection groups(group II).
Results
Among 93 eyes of 87 patients, the injection group consisted of 44 eyes of 41 patients and the non-injection gauge group consisted of 49 eyes of 46 patients. The mean interval between injection and vitrectomy was 5.8 days. Final visual acuity significantly improved as compared to preoperative visual acuity, and group I showed better visual acuity than group II (p=0.008). Visual acuity improved logMAR 0.2 or more in 36 eyes in group I and 43 eyes in group II (p=0.167). The average duration of postoperative vitreous hemorrhage was 1.02 days in group I, and 4.02 days in group II (p=0.2.08). Recurrence of vitreous hemorrhage was not observed in group I or in 2 eyes of group II (p=0.274). Epiretinal membrane occurred in 2 eyes of group I, and in 9 eyes of group II (p=0.031). Only a single eye in group I had neovascular glaucoma after vitrectomy (p=0.527). The operation time of group I was 64.8 minutes, which was significantly shorter than 78.1 minutes of group II (p=0.018).
Conclusions
Intravitreal bevacizumab injection before vitrectomy in proliferative diabetic retinopathy facilitated removal of the fibrovascular membrane, and leads to less postoperative complications and better functional outcomes. Intravitreal bevacizumab injection before vitrectomy can be considered as an effective preoperative adjuvant.
Go to : 
References
1. Flynn HW Jr, Chew EY, Simons DE, et al. Pars plana vitrectomy in the Early Treatment Diabetic Retinopathy Study. ETDRS report number 17. Ophthalmology. 1992; 99:1351–7.
2. Machemer R, Buettner H, Norton EW. Vitrectomy: a pars plana approach. Trans am Acad Ophthalmol Otolaryngol. 1971; 75:813–20.
3. Smiddy WE, Flynn HW. Vitrectomy in the management of diabetic retinopathy. Surv Ophthalmol. 1999; 43:491–507.

4. Sonoda KH, Sakamoto T, Enaida H, et al. Residual vitreouscortex after surgical posterior vitreous separation visualized by intravitreous triamcinolone acetonide. Am J Ophthalmol. 2004; 111:226–30.
5. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006; 113:363–72.

6. Nguyen QD, Shah S, Tatlipinar S, et al. Bevacizuamb suppresses choroidal neovascularization caused by pathological myopia. Br J Ophthalmol. 2008; 89:1368–70.
7. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patient with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331:1480–7.
8. Oshima Y, Sakaguchi H, Gomi F, Tano Y. Regression of iris neovascularization after intravitreal injection of bevacizumab in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2006; 142:155–8.

9. Chen E, Park CH. Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina. 2006; 26:699–700.

10. Maeng HS, Kim JC, Kee CW. Intraviteal Bevacizumab (Avastin® Injection for the Treatment of Early-Stage Neovascular Glaucoma. J Korean Ophthalmol Soc. 2008; 49:696–700.
12. Jonas JB, Hayler JK, Sofker A, Panda-Jonas S. Intravitreal injection of crystalline cortisone as adjunctive treatment of proliferative diabetic retinopathy. Am J Ophthalmol. 2001; 131:468–71.

13. Kim J-Y, Kweon EY, Lee DW, Cho NC. Intravitreal triamcinolone injection with or without bevacizumab for diabetic macular edema. J Korean Ophthalmol Soc. 2008; 49:1275–82.

14. Spaide RF, Fisher YL. Intravitreal bevacizumab(Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006; 26:275–8.
15. Minnella AM, Savastano CM, Ziccardi L, et al. Intravitreal bevacizumab (Avastin®) in proliferative diabetic retinopathy. Acta Ophthalmol. 2008; 86:683–7.
16. Ishikawa K, Honda S, Tsukahara Y, Negi A. Preferable use of intravitreal bevacizumab as a pretreatment of vitrectomy for severe proliferative diabetic retinopathy. Eye. 2009; 23:108–11.

17. Rizzo S, Genovesi-Ebert F, Di Bartolo E, et al. Injection of intravitreal bevacizumab (Avastin) as a preoperative adjunct before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy (PDR). Graefes Arch Clin Exp Ophthalmol. 2008; 246:837–42.

18. Schultz PN, Sobol WM, Weingeist TA. Long-term visual outcome in Terson syndrome. Ophthalmology. 1991; 98:1814–9.

19. Weingeist TA, Goldman EJ, Folk JC, et al. Terson's syndrome. Clinicopathologic correlations. Ophthalmology. 1986; 93:1435–42.
20. Arevalo JF, Maia M, Flynn HW Jr, et al. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol. 2008; 92:213–6.

Go to : 
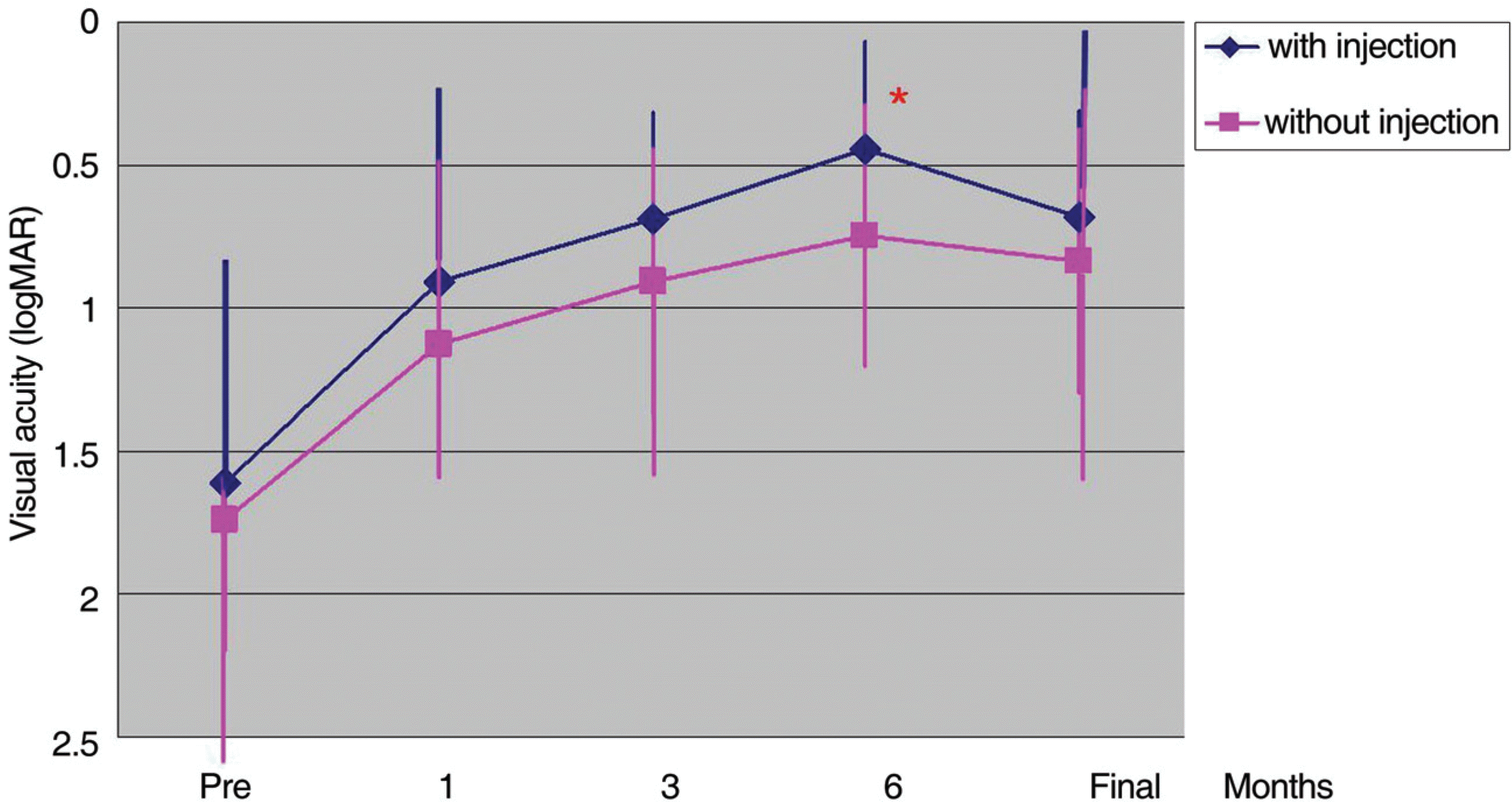 | Figure 1.Average visual acuity changes after vitrectomy with and without pre-operative intravitreal bevacizumab injection for proliferative diabetic retinopathy at baseline and over a 6-month period (* p=0.008, Student t-test). |
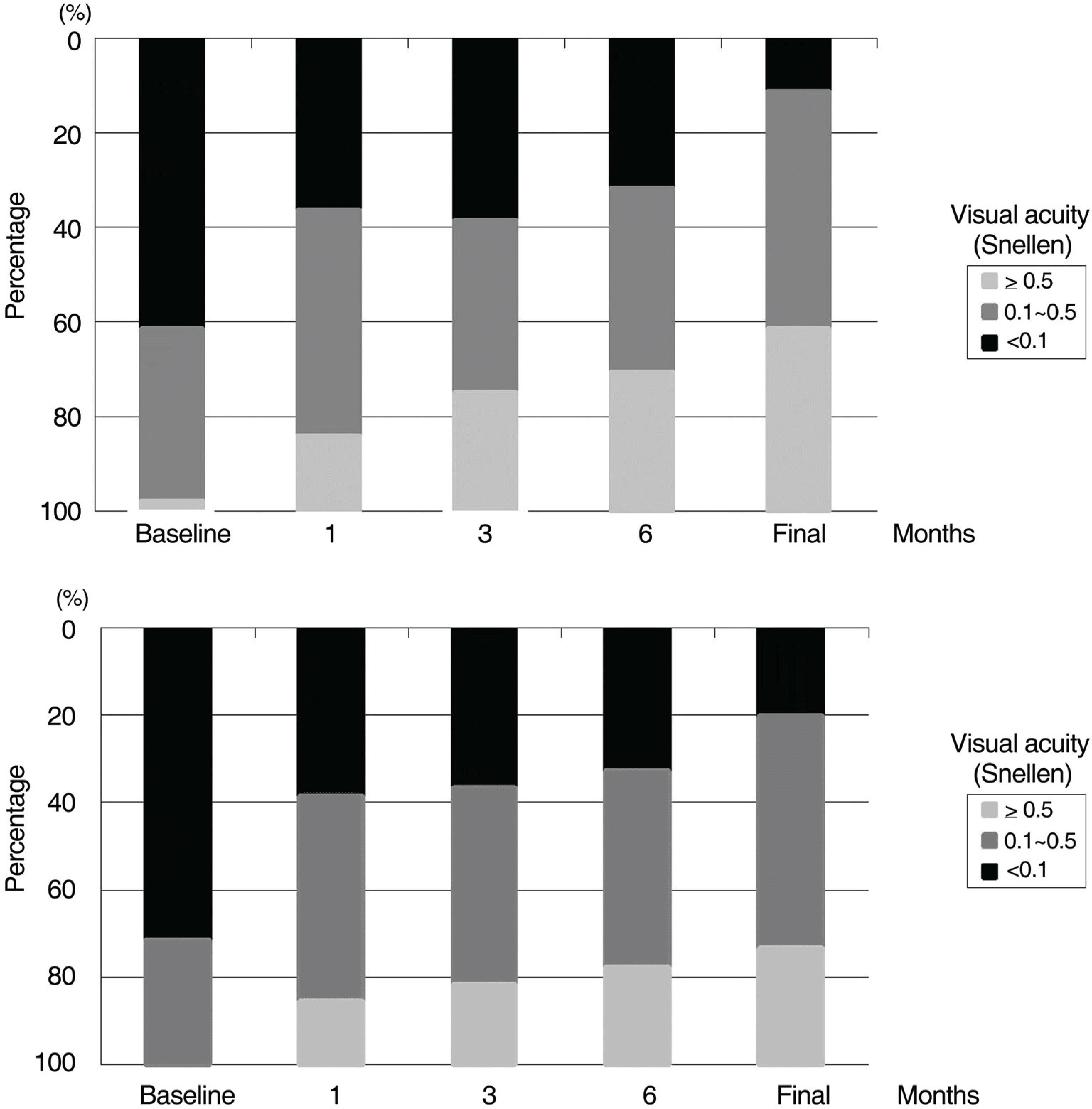 | Figure 2.Visual acuity distribution in proliferative diabetic retinopathy with (A) and without (B) preoperative bevacizumab injection at baseline and overa 6-month period after vitrectomy. |
Table 1.
Baseline characteristics of the patients.
| | Group Ⅰ | Group Ⅱ | p |
|---|---|---|---|
| No. of eyes (patients) | 44 (41) | 49(46) | |
| Age (years) | 54.1±9.4 | 55.9±10.1 | 0.36* |
| Sex (male/female) | 18/23 | 28/18 | |
| Preoperative visual acuity (logMAR) | 1.60±0.89 | 1.74±0.83 | 0.36* |
| Preoperative lens status (%) | | | |
| phakia | 25 (56.8%) | 37 (75.5%) | 0.03† |
| pseudophakia | 19 (43.1%) | 12 (24.5%) | 0.03† |
| Surgical indication | | | |
| vitreous hemorrhage | 25 (56.8%) | 30 (61.2%) | 0.15† |
| progressive fibrovasular proliferation | 4 (9.1%) | 5 (10.2%) | 0.26† |
| vitreous hemorrhage and progressive fibrovasular proliferation | 3 (6.8%) | 3 (6.1%) | 0.32† |
| tractional retinal detachment involving macula | 12 (27.3%) | 11 (22.4%) | 0.16† |
| Follow-up (months) | 10.8±3.03 | 12.8±5.97 | 0.06* |
Table 2.
Time of bevacizumab injection before vitrectomy
Table 3.
Comparison of the operation time between with and without preoperative intravitreal bevacizumab injection for proliferative diabetic retinopathy
| | With injection | Without injection | p |
|---|---|---|---|
| Operation time* (total, minutes) | 66.4±25.1 | 78.1±28.7 | 0.018† |
| with cataract operation | 72.6±23.9 (n=23) | 85.6±26.3 (n=33) | 0.062† |
| without cataract operation | 56.4±21.7 (n=21) | 62.5±26.6 (n=16) | 0.462† |
| Operation time (subtype) | | | |
| vitreous hemorrahage | 62.0±19.9 (n=25) | 70.0±24.1 (n=30) | 0.18† |
| progressive fibrovascular proliferation | 57.5±21.8 (n=4) | 84.0±7.41 (n=5) | 0.09† |
| vitreous hemorrhage and progressive fibrovascular proliferation | 71.7±14.4 (n=3) | 100.5±37.0 (n=3) | 0.05† |
| tractional retinal detachment involving macula | 71.6±27.7 (n=12) | 66.6±17.5 (n=11) | 0.81† |
Table 4.
Postoperative complications after vitrectomy for proliferative diabetic retinopathy with and without pre-operative injecton of bevacizumab
| |
No. of cases (%) |
||
|---|---|---|---|
| with injection | without injection | p* | |
| Vitreous hemorrhage recurrence | 0 | 2 (4.08) | 0.274 |
| Neovascular glaucoma | 0 | 1 (2.04) | 0.527 |
| Epiretinal membrane | 2 (4.54) | 9 (18.36) | 0.031 |
| Retinal detachment | 1 (2.27) | 2 (4.08) | 0.398 |
| Choroidal detachment | 1 (2.27) | 0 | 0.473 |
| Total | 4 (9.09) | 14 (28.5) | 0.012 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download