Abstract
Purpose
To report a case of partial third cranial nerve palsy in a patient with suprasellar cysticercosis. Surgical removal of the cyst was followed by symptom improvement.
Case summary
A 36-year-old man presented with binocular diplopia for 3 months. His best corrected visual acuities were 20/20 in both eyes, and both slit lamp and fundus examinations were unremarkable. The alternate prism cover test revealed four prism diopters (Δ) of exotropia and 4Δ of left hypotropia. Supraduction and adduction was mildly limited in the left eye. Pupil size was larger in the left eye and anisocoria was greater under bright light. Color test and visual field examination were normal. Neurologic examination showed a weakness of grade IV in the upper and lower extremities. Brain magnetic resonance imaging revealed a well-encapsulated cystic mass of homogeneous low intensity signal in the suprasellar area extending into the midbrain. Craniotomy and cyst removal were performed, and histologic findings were compatible with neurocysticercosis. Two weeks postoperatively the patient was free of diplopia and limb weakness.
Figures and Tables
Figure 1
Nine gaze photograph of the patient reveals a marginal limitation of elevation of the left eye.

Figure 2
Lancaster red-green test at the first visit revealed a small angle of left hypotropia and exotropia. The solid line represents the view of the left eye and the dotted line represents the view of the right eye. The left hypotropia increased in upgaze and right gaze. The angle of deviation increased with the left eye fixating (secondary deviation).
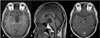
Figure 3
(A) Preoperative T1-weighted axial magnetic resonance image showing a large cystic mass with homogeneous low signal intensity and well-defined borders (indicated by the white arrow), producing a mass effect in the midbrain mainly to the left, within the level of cerebral peduncles and fascicles of the oculomotor nerve in the brainstem. The head and neck of the larvae (scolex) is seen just next to the main mass (indicated by the hollow arrow). (B) Preoperative T1-weighted sagittal magnetic resonance image showing a homogeneous low signal intensity mass with well-defined borders compressing the midbrain and cerebral peduncles. (C) Postoperative gadolinium enhanced T1-weighted axial magnetic resonance image showing complete removal of the large cystic mass and an intact midbrain. There is a focal enhancing lesion just right to the interpeduncular cistern (indicated by the white arrow), most likely to be a post-operative reactive enhancement.
References
1. Garcia HH, Gonzalez AE, Evans CA, Gilman RH. Taenia solium cysticercosis. Lancet. 2003. 362:547–556.
2. Garcia HH, Gonzalez AE, Gilman RH. Diagnosis, treatment and control of Taenia solium cysticercosis. Curr Opin Infect Dis. 2003. 16:411–419.
3. Pandey PK, Chaudhuri Z, Bhatia A. Extraocular muscle cysticercosis presenting as Brown syndrome. Am J Ophthalmol. 2001. 131:526–527.
4. Mohan K, Saroha V, Sharma A, et al. Extraocular muscle cysticercosis: clinical presentations and outcome of treatment. J Pediatr Ophthalmol Strabismus. 2005. 42:28–33.
5. Ko EK, Kim JP, Ko CJ. A case of cysticercosis in lateral rectus muscle. J Korean Ophthalmol Soc. 1975. 16:82–84.
6. Boecher-Schwarz HG, Hey O, Higer HP, Perneczky A. Intrasellar cysticercosis mimicking a pituitary adenoma. Br J Neurosurg. 1991. 5:405–407.
7. Rafael H, Gomez-Llata S. Intrasellar cysticercosis. Case report. J Neurosurg. 1985. 63:975–976.
8. Singhi P, Mahajan V, Khandelwal NK. Sudden-onset ptosis caused by midbrain neurocysticercosis in 2 children. J Child Neurol. 2008. 23:334–337.
9. Kim JS, Jeong SM, Moon SY, Park SH. Third cranial nerve palsy from midbrain neurocysticercosis: repeated exacerbation on tapering corticosteroids. J Neuroophthalmol. 2004. 24:217–220.
10. Chotmongkol V, Sawanyawisuth K, Limpawattana P, et al. Superior divisional oculomotor nerve palsy caused by midbrain neurocysticercosis. Parasitol Int. 2006. 55:223–225.
11. Takahashi M, Kase M, Suzuki Y, et al. Incomplete oculomotor palsy with pupil sparing caused by compression of the oculomotor nerve by a posterior communicating posterior cerebral aneurysm. Jpn J Ophthalmol. 2007. 51:470–473.
12. Kim JS, Kim J. Pure midbrain infarction: clinical, radiologic, and pathophysiologic findings. Neurology. 2005. 64:1227–1232.
13. White AC, Garcia HH. Recent developments in the epidemiology, diagnosis, treatment, and prevention of neurocysticercosis. Curr Infect Dis Rep. 1999. 1:434–440.
14. Martinez HR, Rangel-Guerra R, Arredondo-Estrada JH, et al. Medical and surgical treatment in neurocysticercosis a magnetic resonance study of 161 cases. J Neurol Sci. 1995. 130:25–34.
15. Garcia HH, Gilman RH, Horton J, et al. Cysticercosis Working Group in Peru. Albendazole therapy for neurocysticercosis: a prospective double-blind trial comparing 7 versus 14 days of treatment. Neurology. 1997. 48:1421–1427.
16. Colli BO, Carlotti CG Jr, Assirati JA Jr, et al. Surgical treatment of cerebral cysticercosis: long-term results and prognostic factors. Neurosurg Focus. 2002. 12:3.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download