Abstract
Purpose
To evaluate the short-term effect of intravitreal bevacizumab (AvastinⓇ) in polypoidal choroidal vasculopathy. Methods: Intravitreal AvastinⓇ was injected into 13 eyes of 13 patients with PCV in this retrospective, interventional case study. The follow-up period lasted over 3 months after therapy. Changes in best-corrected visual acuity (BCVA), foveal height determined by optical coherence tomography, and abnormal vasculature in indocyanine green angiography (ICGA) were evaluated.
Results
The mean LogMAR BCVA was 0.82 at baseline, 0.78 at 1 month after treatment, and 0.73 at 3 months after treatment. Visual acuity was stabilized or improved in 13 eyes (100%). The mean foveal height was 288 µm at baseline, 231 µm (p<0.05) at 1 month after treatment, and 196 µm at 3 months after treatment. The polypoidal lesions in ICGA decreased in 4 eyes (31%), although branching vasculature in ICGA was unchanged in 13 eyes (100%).
Conclusions
Intravitreal injection of AvastinⓇ may stabilize visual acuity and reduce macular edema due to decreased retinal pigment epithelial detachment and leaking. However, intravitreal injection had a minimal effect in occlusion of the symptomatic polypoidal lesions and no effect in occlusion of the branching vascular network.
Go to : 
References
1. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990; 10:1–8.

2. Ciardella AP, Donsoff IM, Huang SJ. . Polypoidal choroidal vasculopathy. Surv Ophthalmol. 2004; 49:25–37.

3. Uyama M, Matsubara T, Fukushima I. . Idiopathic polypoidal vasculopathy in Japanese patients. Arch Ophthalmol. 1999; 117:1035–42.
4. Uyama M, Wada M, Nagai Y. . Polypoidal choroidal vasculopathy: Natural history. Am J Ophthalmol. 2002; 133:639–48.
5. Lee WK, Kwon SI. Polypoidal choroidal vasculopathy. J Korean Ophthalmol Soc. 2000; 42:2573–84.
6. Scassellati-Sforzolini B, Mariotti C, Bryan R. . Polypoidal choroidal vasculopathy in Italy. Retina. 2001; 21:121–5.

7. Lafaut BA, Leys AM, Snyers B. . Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol. 2000; 238:752–9.

8. Wen F, Chen C, Wu D, Li H. Polypoidal choroidal vasculopathy in elderly Chinese patients. Graefes Arch Clin Exp Ophthalmol. 2004; 242:625–9.

9. Sho K, Takahashi K, Yamada H. . Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003; 121:1392–6.
10. Lee JW, Kim IT. Epidemiologic and clinical characteristics of polypoidal choroidal vasculopathy in Korean patients. J Korean Ophthalmol Soc. 2006; 48:63–74.
11. Rosa RH Jr. Davis JL, Eifrig CW. Clinicopathologic reports, case reports, and small case series: clinicopathologic correlation of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol. 2002; 120:502–8.
12. Kuroiwa S, Tatiwa H, Hisatomi T. . Pathological features of surgically excised polypoidal choroidal vasculopathy membranes. Clin Experiment Ophthalmol. 2004; 32:297–302.

13. Yuzawa M, Mori R, Kawamura A. The origins of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2005; 89:602–7.

14. Tong JP, Chan WM, Liu DT. . Aqueous humor levels of vascular endothelial growth factor and pigment epitheliumderived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol. 2006; 141:456–62.

15. Matsuoka M, Ogata N, Otsugi T. . Expression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol. 2004; 88:809–15.

16. Yuzawa M, Mori R, Haruyama M. A study of laser photocoagulation for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2003; 47:379–84.

17. Gomi F, Ohji M, Sayanagi K. . 1-Year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology. 2008; 115:141–6.
18. Lee PY, Kim KS, Lee WK. Photodynamic therapy with verteporfin in polypoidal choroidal vasculopathy. J Korean Ophthalmol Soc. 2004; 45:216–27.
19. Rich RM, Rosenfeld PJ, Puliaflilo GA. . Short term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina. 2006; 26:495–511.
20. Spaide RF, Laud K, Fine HF. . Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006; 26:383–90.

21. Lee SC, Seong YS, Kim SS. . Photodynamic therapy with vertepofin for polypoidal choroidal vasculopathy of the macula. Ophthalmologica. 2004; 218:193–201.
22. Chan WM, Lam DC, Lai TY. . Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy. Ophthalmology. 2004; 111:1579–84.

23. Silva RM, Figueira J, Cachulo ML. . Polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Graefe Arch Clin Exp Ophthalmol. 2005; 243:973–9.

24. Iijima H, Iida T, Imai M. . Optical coherence tomography of orange-red subretinal lesions in eyes with idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol. 2000; 129:21–6.

25. Yang JC, Haworth L, Sherry RM. . A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003; 349:427–34.

26. Hurwitz H, Fehrenbacher L, Novotny W. . Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004; 350:2335–42.

27. Ghajarnia M, Kurup S, Eller A. The therapeutic effects of intravitreal bevacizumab in a patient with recalcitrant idiopathic polypoidal choroidal vasculopathy. Semin Ophthalmol. 2007; 22:127–31.

28. Gomi F, Sawa M, Sakaguchi H. . Efficacy of intravitreal bevacizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol; 2008; 92:70–3.

29. Shahar J, Avery RL, Heilweil G. . Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin). Retina. 2006; 26:262–9.

30. Heiduschka P, Julien S, Hofmeister S. . Bevacizumab (avastin) dose not harm retinal function after intravitreal injection as shown by electroretinography in adult mice. Retina. 2008; 28:46–55.
Go to : 
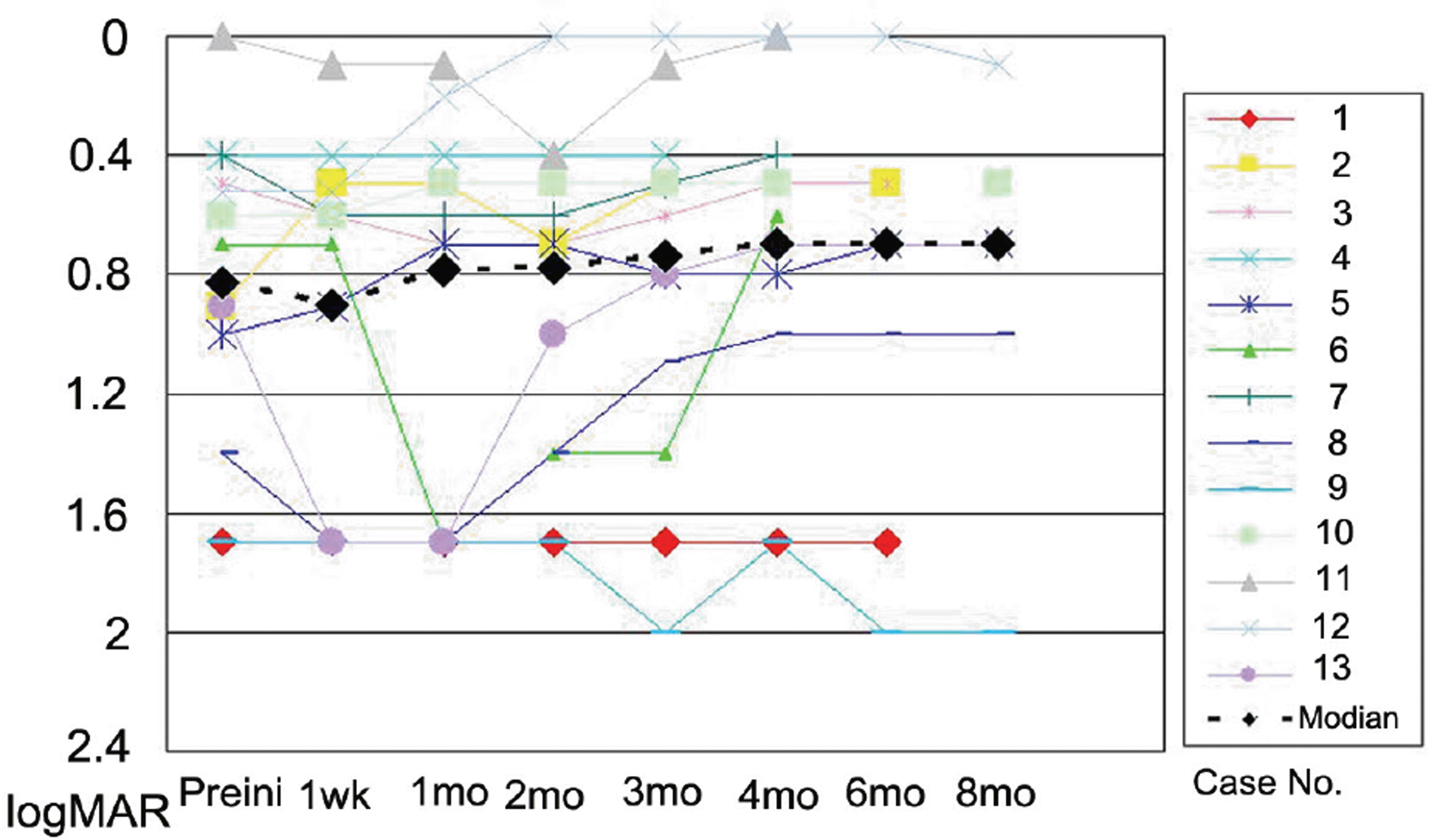 | Figure 1.Change in logarithm of the minimum angle of resolution (LogMAR) best-corrected visual acuity (VA) with time (months). Improvements of three or more lines of VA were detected in 2 eyes (case 6 and 8). |
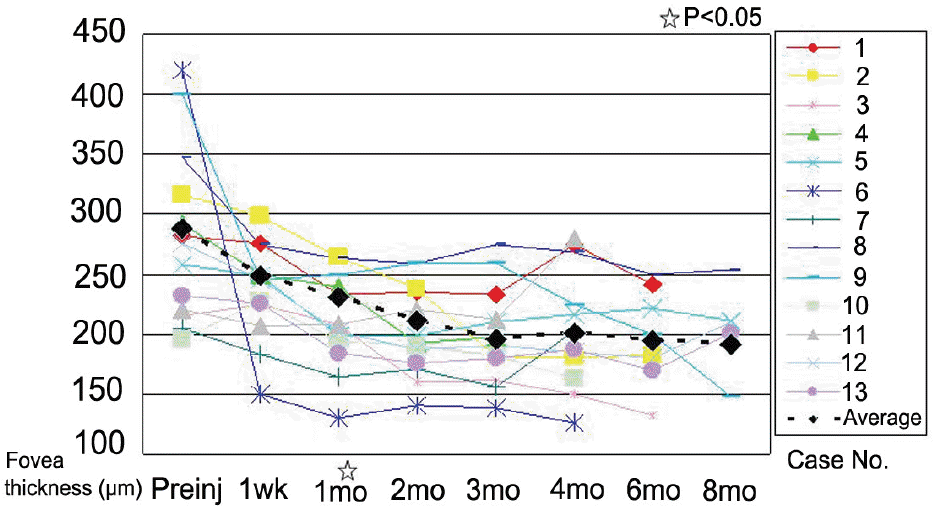 | Figure 2.Change in mean fovea thickness with time (months). A significant decrease was found at 1 month (p<0.05). |
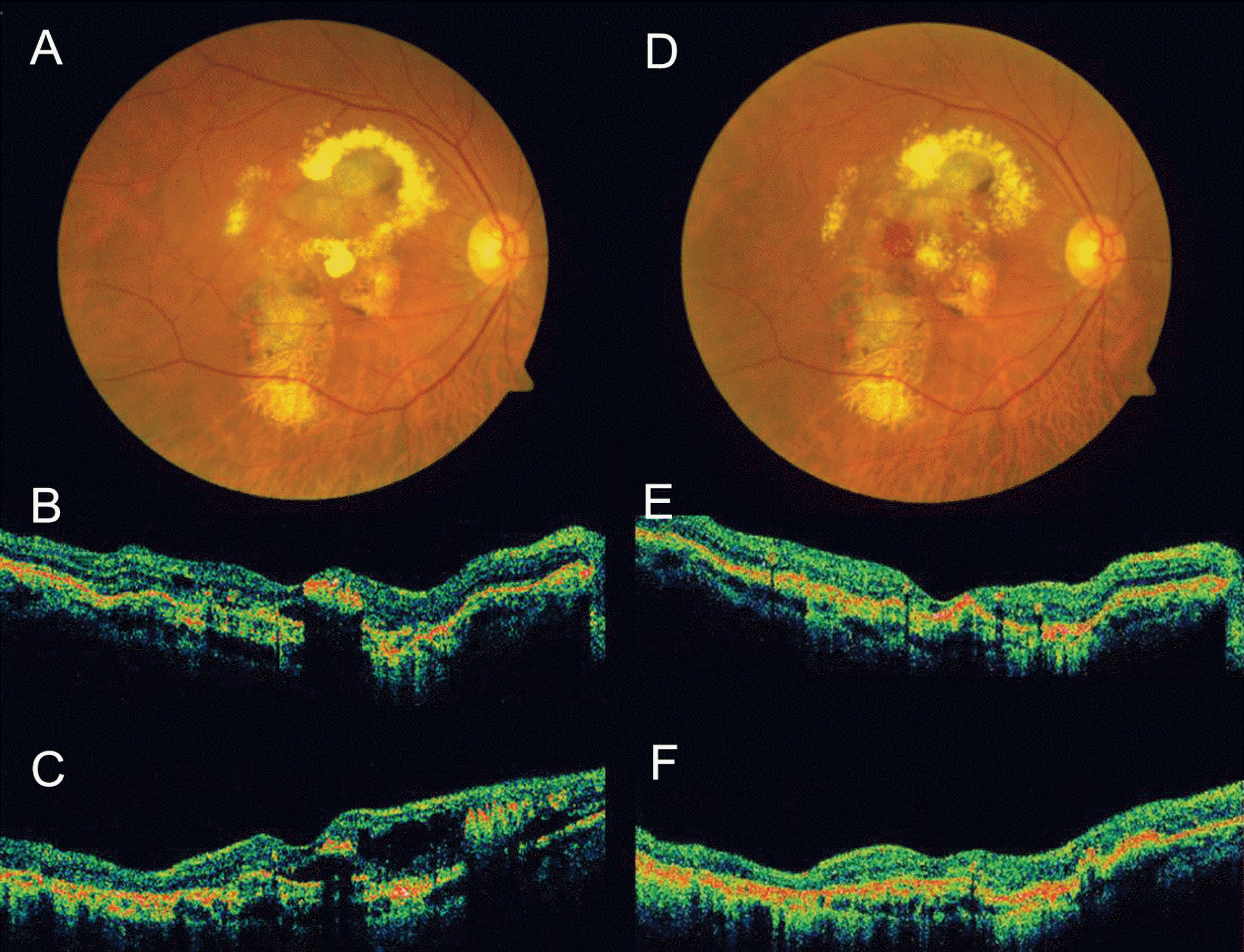 | Figure 3.Case No. 3 (A) Baseline fundus photograph shows hard exudates, pigmentations around macula. (B) Baseline horizontal OCT image and (C) vertical OCT image shows protruding polyps and macular edema. (D)~(F) One month after the second injection, macular edema reduced, but subretinal hemorrhage occurred. |
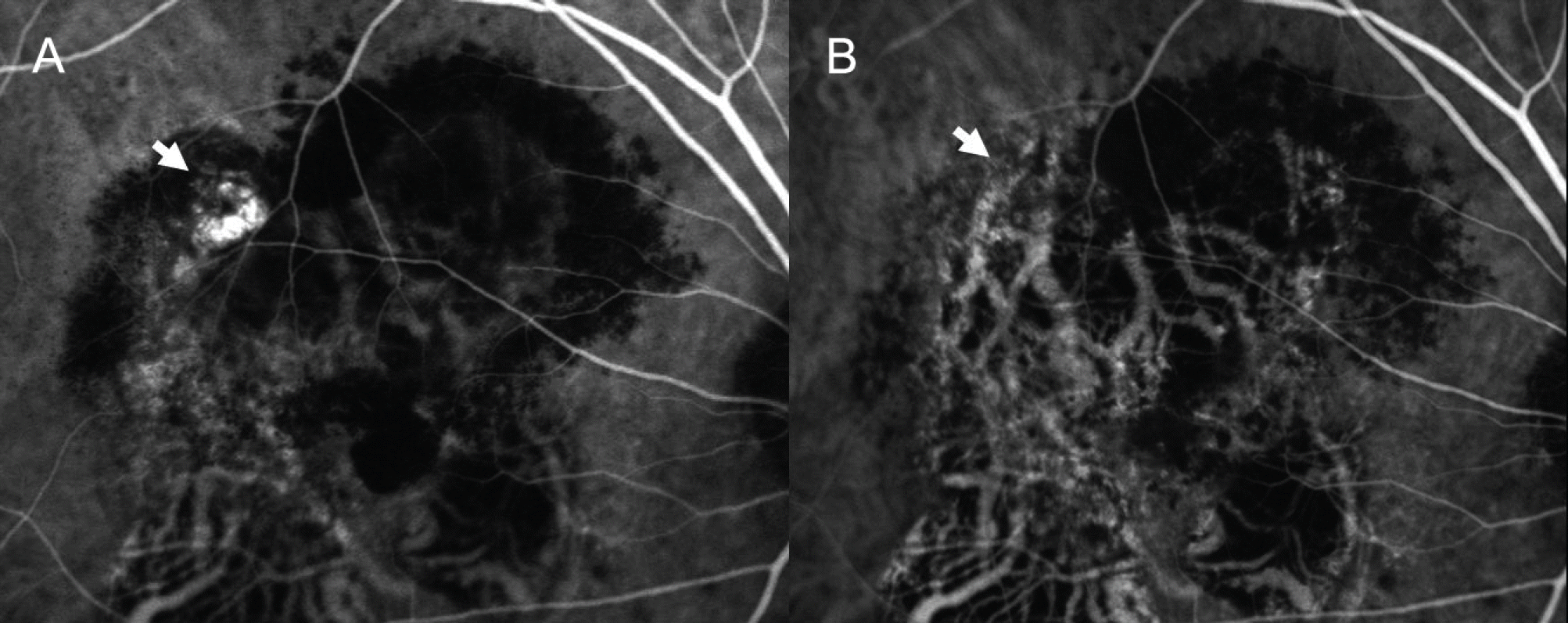 | Figure 4.Case No. 3 (A) Baseline ICGA shows polypoidal dilatation of choroidal vessels and branching vascular network. (B) ICGA of one month after the second injection, the actively stained polyps resolve completely (arrows) while branching vascular network remains. |
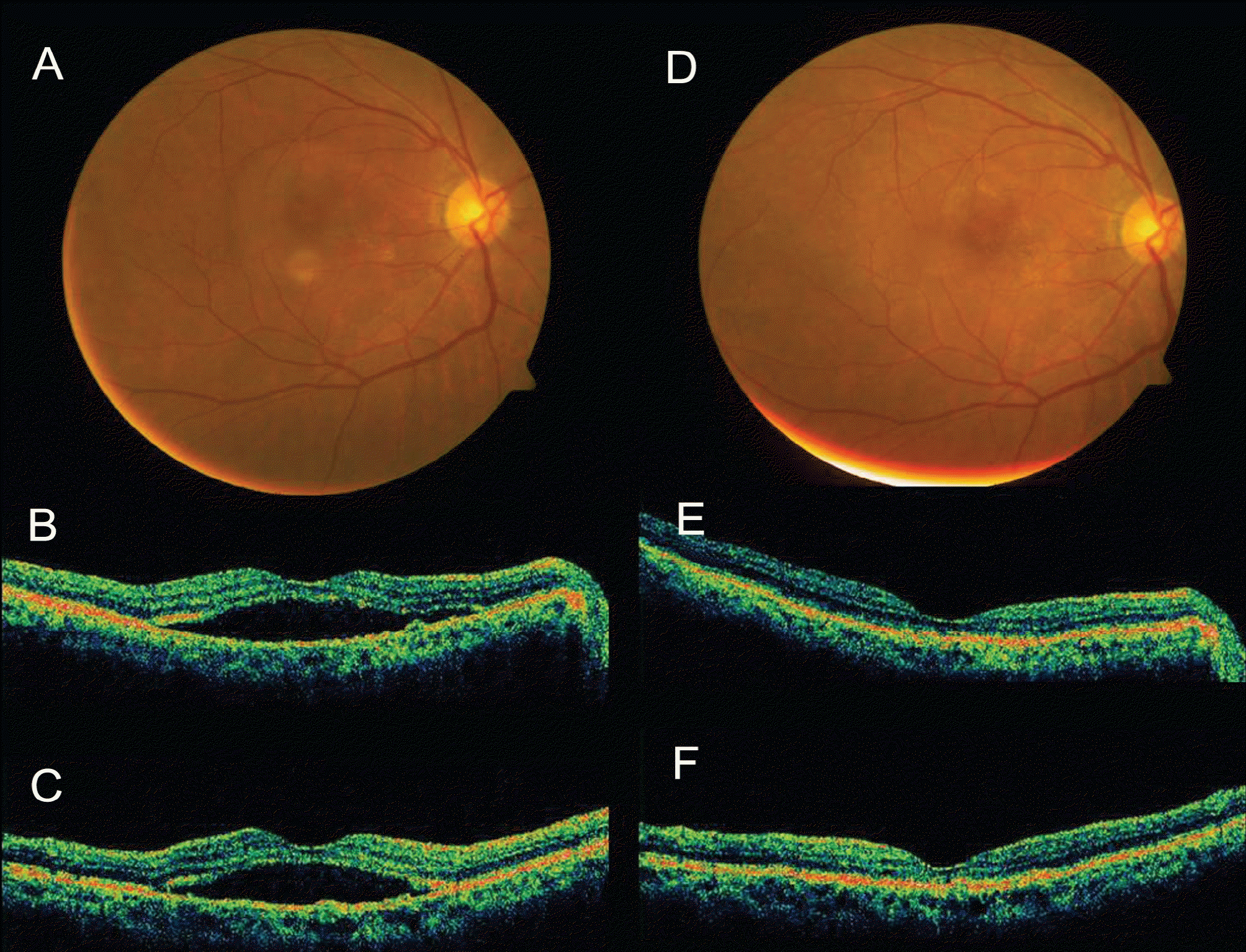 | Figure 5.Case No. 6 (A) Baseline fundus photograph shows orange nodular lesion at temporal area from fovea. (B) Baseline horizontal OCT image and (C) vertical OCT image shows subretinal fluid. (D~F) One month after the third injection, subretinal fluid resolved. |
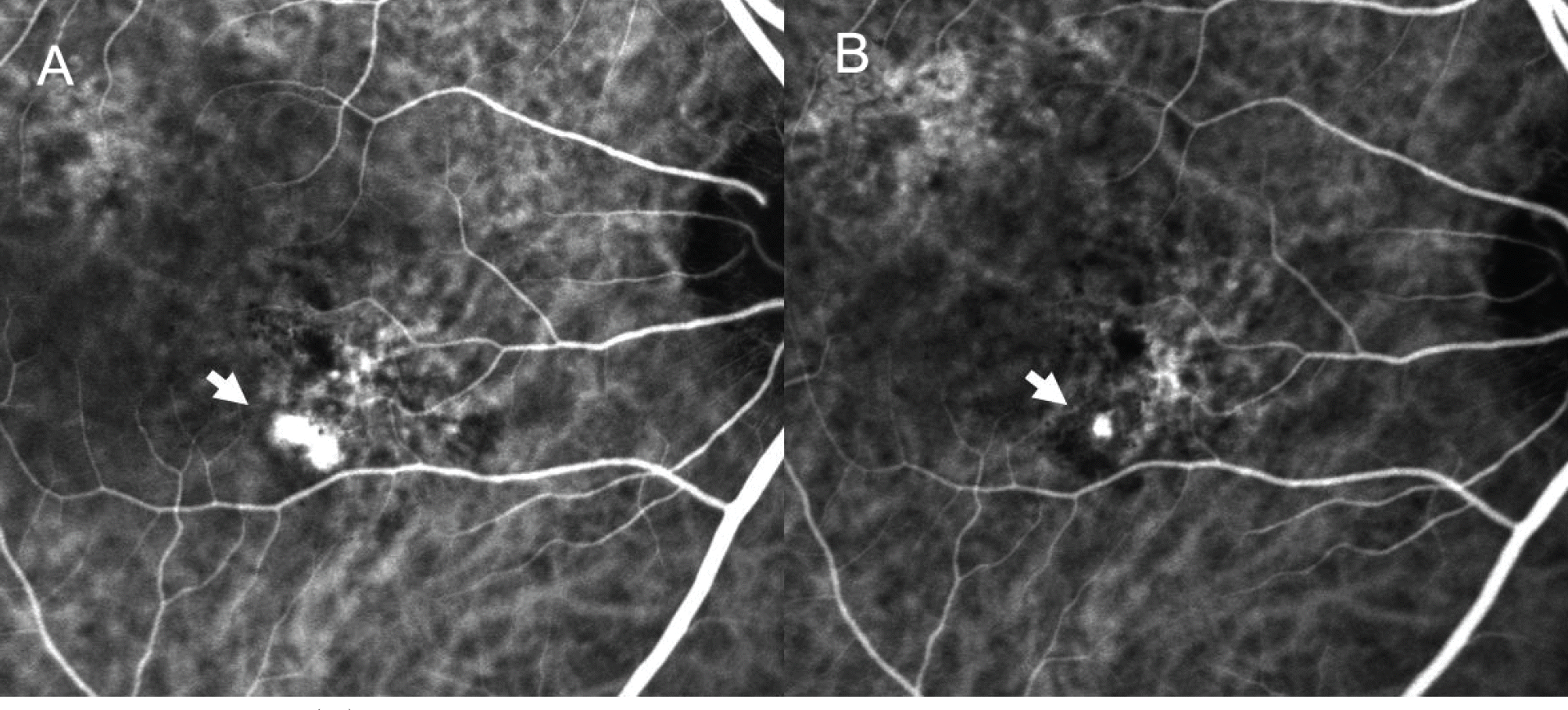 | Figure 6.Case No. 6 (A) Baseline ICGA shows polypoidal dilatation of choroidal vessels and branching vascular network. (B) ICGA one month after the third injection, the polyps resolved incompletely (arrows). |
 | Figure 7.Case No. 12 (A) Baseline fundus photograph shows orange nodular lesion at nasal area from fovea. (B) Baseline horizontal OCT image and (C) vertical OCT image shows multiple PED and polyps. (F~H) One month after the second injection, PED reduced. (D) Baseline FA shows leaking at corresponding region of the fundus, and pooling at PED region. (I) FA of one month after the second injection shows reduced leaking. (E) Baseline ICGA shows polypoidal dilatation of choroidal vessels (arrows). (J) ICGA of one month after the second injection, the vascular findings did not change. |
 | Figure 8.Case No. 12 (A) Fundus photograph of seven month after the second injection. (B) Horizontal OCT image and (C) vertical OCT image shows increased PED. (D~ F) One month after the third injection, PED reduced. |
 | Figure 9.Case No. 12 (A) Baseline ICGA shows hidden area due to hemorrhage PED (broken arrows). (B) ICGA of one month after the second injection, the vascular findings did not change (arrows). (C) ICGA of one month after the third injection shows increased polypoidal lesion (arrowhead). |
 | Figure 11.Case No. 13 (A) Baseline ICGA shows polypoidal dilatation of choroidal vessels hidden area due to hemorrhage PED (arrows). (B) ICGA of one month after the second injection, the polyps remain, but activity decreased. (C) ICGA of six month after the second injection shows increased polypoidal lesion (arrowhead). |
 | Figure 10.Case No. 13 (A) Baseline fundus photograph shows orange nodular lesion around macula. (B) Baseline horizontal OCT image and (C) vertical OCT image shows PED, polyp and subretinal fluid (D~F) One month after the second injection, PED and subretinal fluid reduced. (G~ I) Six month after the second injection, subretinal fluid increased. |
Table 1.
Patient characteristics and clinical date before and after intravitreal injection of bevacizumab for PCV
| Case No. | Age | Sex | Previous PDT∗ | Pre Tx BCVA† | Post Tx BCVA | Pre Tx fovea thickness(µm) | Post Tx fovea thickness(µm) | Polyp closure | Branching network closure | F/U mo | No of reinjection |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 81 | F | Yes | 0.02 | 0.02 | 282 | 241 | No | No | 6 | 2 |
| 2 | 72 | F | No | 0.125 | 0.32 | 316 | 182 | Yes | No | 6 | 3 |
| 3 | 70 | M | No | 0.32 | 0.32 | 215 | 132 | No | - | 5 | 2 |
| 4 | 59 | M | No | 0.2 | 0.2 | 292 | 198 | No | No | 3 | 0 |
| 5 | 58 | M | Yes | 0.1 | 0.2 | 258 | 211 | Yes | No | 8 | 3 |
| 6 | 74 | M | No | 0.2 | 0.25 | 420 | 126 | Yes | No | 4 | 2 |
| 7 | 64 | F | No | 0.4 | 0.32 | 205 | 203 | No | No | 4 | 1 |
| 8 | 64 | M | Yes | 0.04 | 0.02 | 347 | 254 | No | No | 8 | 1 |
| 9 | 70 | F | Yes | 0.02 | 0.02 | 400 | 148 | No | No | 8 | 3 |
| 10 | 83 | F | No | 0.25 | 0.32 | 196 | 163 | No | No | 4 | 2 |
| 11 | 59 | M | No | 1.0 | 1.0 | 220 | 280 | Yes | No | 4 | 2 |
| 12 | 68 | M | No | 0.3 | 0.6 | 245 | 210 | No | No | 8 | 2 |
| 13 | 73 | F | No | 0.125 | 0.16 | 232 | 201 | No | - | 8 | 1 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download