Abstract
Purpose
To evaluate the effects of TGF-β inhibitor on the wound healing process after corneal laceration, and its inhibitory effect on corneal scar formation.
Methods
Forty Lewis rats were randomly divided into one control and three experimental groups (groups I, II, and III). After partial-thickness vertical linear corneal incision, a diluted solution with 10, 25, and 50 μ g of TGF-β inhibitor was instilled into each eye of groups I, II, and III respectively. Corneal haze was measured by using slit-lamp biomicroscopic examination. Using histopathologic examination, we compared the number of stromal keratocytes and the arrangement of regenerated collagen fibers. We also performed immunohistochemistry to confirm the differential expression of fibronectin and α-smooth muscle actin in each group.
Go to : 
References
1. Krachmer JH, Mannis MJ, Holland EJ. Cornea. 2nd ed. Philadelphia: Elsevier & Mosby;2005. p. 115–46.
2. Ohji M, SundarRaj N, Thoft RA. Transforming growth factor-beta stimulates collagen and fibronectin synthesis by human corneal stromal fibroblasts in vitro. Curr Eye Res. 1993; 12:703–9.
3. Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM. Induction of alpha-smooth muscle actin expression and myofibroblast transfo-rmation in cultured corneal keratocytes. Cornea. 1996; 15:505–16.
4. Fini ME, Girard MT, Matsubara M, Bartlett JD. Unique regulation of the matrix metalloproteinase, gelatinase B. Invest Ophthalmol Vis Sci. 1995; 36:622–33.
5. Grant MB, Khaw PT, Schultz GS, et al. Effects of epidermal growth factor, fibroblast growth factor, and transforming growth factor-beta on corneal cell chemotaxis. Invest Ophthalmol Vis Sci. 1992; 33:3292–301.
6. Desmouliere A, Rubbia-Brandt L, Grau G, Gabbiani G. Heparin induces alpha-smooth muscle actin expression in cultured fibroblasts and in granulation tissue myofibroblasts. Lab Invest. 1992; 67:716–26.
7. Garana RM, Petroll WM, Chen WT, et al. Radial keratotomy. II. Role of the myofibroblast in corneal wound contraction. Invest Ophthalmol Vis Sci. 1992; 33:3271–82.
8. Petroll WM, Cavanagh HD, Barry P, et al. Quantitative analysis of stress fiber orientation during corneal wound contraction. J Cell Sci. 1993; 104:353–63.

9. Shah M, Foreman DM, Ferguson MW. Neutralizing antibody to TFG-beta 1,2 reduces cutaneous scarring in adult rodents. J Cell Sci. 1994; 107:1137–57.
10. Cordeiro MF, Mead A, Ali RR, et al. Novel antisense oligo-nucleotides targeting TGF-beta inhibit in vivo scarring and improve surgical outcome. Gene Ther. 2003; 10:59–71.
11. Cordeiro MF, Gay JA, Khaw PT. Human anti-transforming growth factor-beta2 antibody: a new glaucoma anti-scarring agent. Invest Ophthalmol Vis Sci. 1999; 40:2225–34.
12. Siriwardena D, Khaw PT, King AJ, et al. Human antitransforming growth factor beta (2) monoclonal antibody-a new modulator of wound healing in trabeculectomy: a randomized placebo controlled clinical study. Ophthalmology. 2002; 109:427–31.
13. Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Neutralizing antibody to TGF-beta modulates stromal fibrosis but not regression of photoablative effect following PRK. Curr Eye Res. 1998; 17:736–47.
14. Mathers WD, Lemp MA. Theevolution of scarring following penetrating keratoplasty. Cavanogh HD, editor. The cornea: Trans-actions of the World congress on the Cornea III. New York: Raven Press;1988. Ⅱ:chap. 16.
15. Goodman GL, Trokel SL, Stark WJ, et al. Corneal healing following laser refractive keratectomy. Arch Ophthalmol. 1989; 107:1799–803.

16. Thompson NL, Bazoberry F, Speir EH, et al. Transforming growth factor beta-1 in acute myocardial infarction in rats. Growth Factors. 1988; 1:91–9.

17. Wahl SM. Transforming growth factor beta (TGF-beta) in inflammation: a cause and a cure. J Clin Immunol. 1992; 12:61–74.
18. Mita T, Yamashita H, Kaji Y, et al. Effects of transforming growth factor beta on corneal epithelial and stromal cell function in a rat wound healing model after excimer laser keratectomy. Graefes Arch Clin Exp Ophthalmol. 1998; 236:834–43.
19. Hayashi K, Frangieh G, Wolf G, Kenyon KR. Expression of transforming growth factor-beta in wound healing of vitamin A-deficient rat corneas. Invest Ophthalmol Vis Sci. 1989; 30:239–47.
20. Rochels R, Busse WD. In vivo evidence for the chemotactic activity of cyclooxygenase- and lipoxygenase-dependent compounds using a corneal implantation technique. Ophthalmic Res. 1984; 16:194–7.

21. Azar DT, Hahn TW, Jain S, et al. Matrix metalloproteinases are expressed during wound healing after excimer laser keratectomy. Cornea. 1996; 15:18–24.

22. Rieck P, Assouline M, Savoldelli M, et al. Recombinant human basic fibroblast growth factor (Rh-bFGF) in three different wound models in rabbits: corneal wound healing effect and pharmacology. Exp Eye Res. 1992; 54:987–98.

23. Ohno K, Mitooka K, Nelson LR, et al. Keratocyte activation and apoptosis in transplanted human corneas in a xenograft model. Invest Ophthalmol Vis Sci. 2002; 43:1025–31.
24. Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999; 18:529–51.

25. Eckes B, Kessler D, Aumailley M, Krieg T. Interactions of fibroblasts with the extracellular matrix: implications for the understanding of fibrosis. Springer Semin Immunopathol. 1999; 21:415–29.

26. Davison PF, Galbavy EJ. Fluorescent dyes demonstrate the uniform expansion of the growing rabbit cornea. Invest Ophthalmol Vis Sci. 1985; 26:1202–9.
27. Davison PF, Galbavy EJ. Connective tissue remodeling in corneal and scleral wounds. Invest Ophthalmol Vis Sci. 1986; 27:1478–84.
28. Tuft SJ, Zabel RW, Marshall J. Corneal repair following keratectomy. A comparison between conventional surgery and laser photoablation. Invest Ophthalmol Vis Sci. 1989; 30:1769–77.
29. Jain S, Hahn TW, McCally RL, Azar DT. Antioxidants reduce corneal light scattering after excimer keratectomy in rabbits. Lasers Surg Med. 1995; 17:160–5.

30. Kim TI, Lee SY, Pak JH, et al. Mitomycin C, ceramide, and 5-fluorouracil inhibit corneal haze and apoptosis after PRK. Cornea. 2006; 25:55–60.

31. Carones F, Vigo L, Scandola E, Vacchini L. Evaluation of the prophylactic use of mitomycin-C to inhibit haze formation after photorefractive keratectomy. J Cataract Refract Surg. 2002; 28:2088–95.

Go to : 
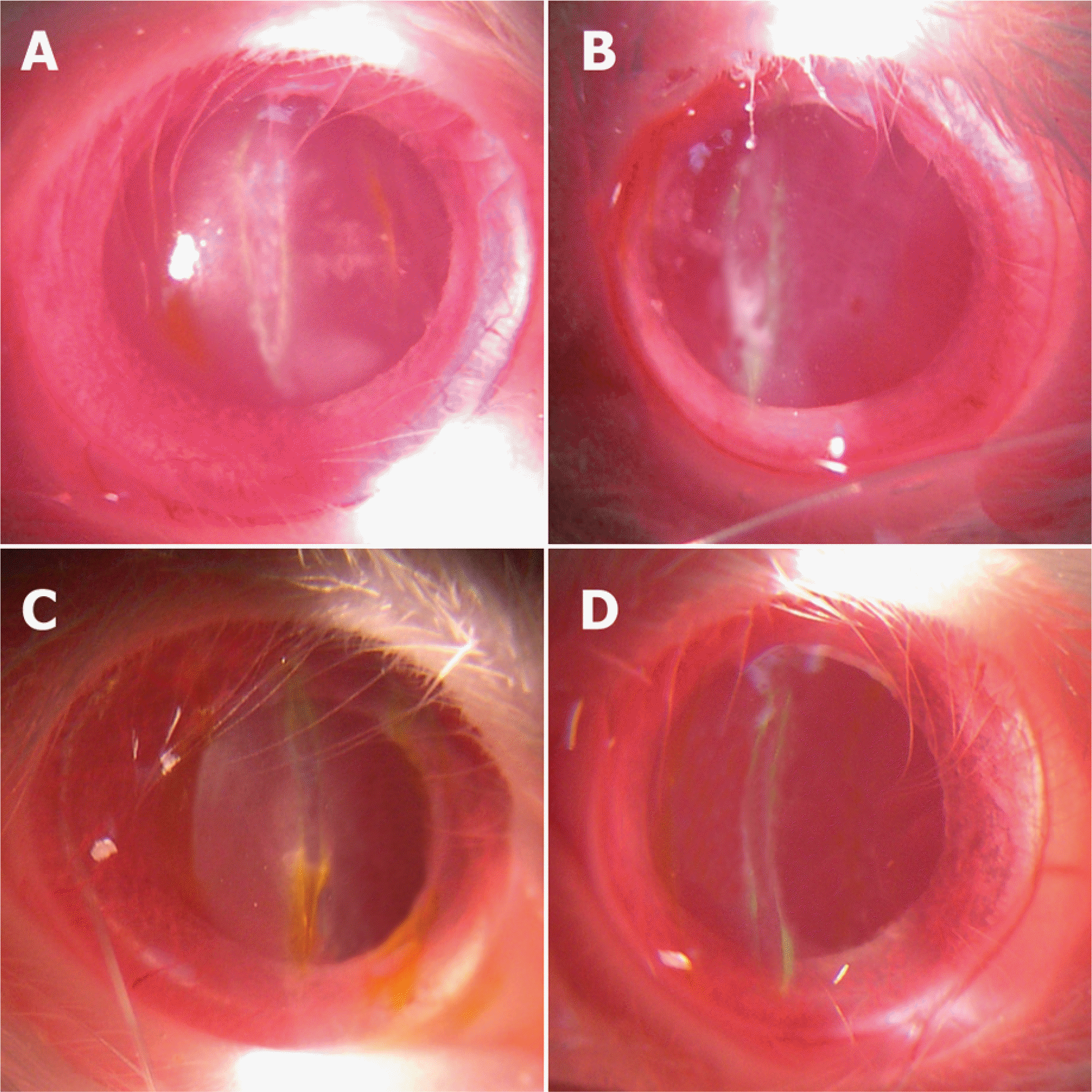 | Figure 1.Slit-lamp biomicroscopic photographs of the corneas at 2 weeks after corneal laceration. (A) Control group (B) Group I (treated with TGF-β inhibitor 10 μg) (C) Group II (treated with TGF-β inhibitor 25 μg) (D) Group III (treated with TGF-β inhibitor 50 μg). Linear corneal opacities were noted in the center of the cornea the severity of the corneal opacity in group II and group III was less severe than that in the control group and group I. |
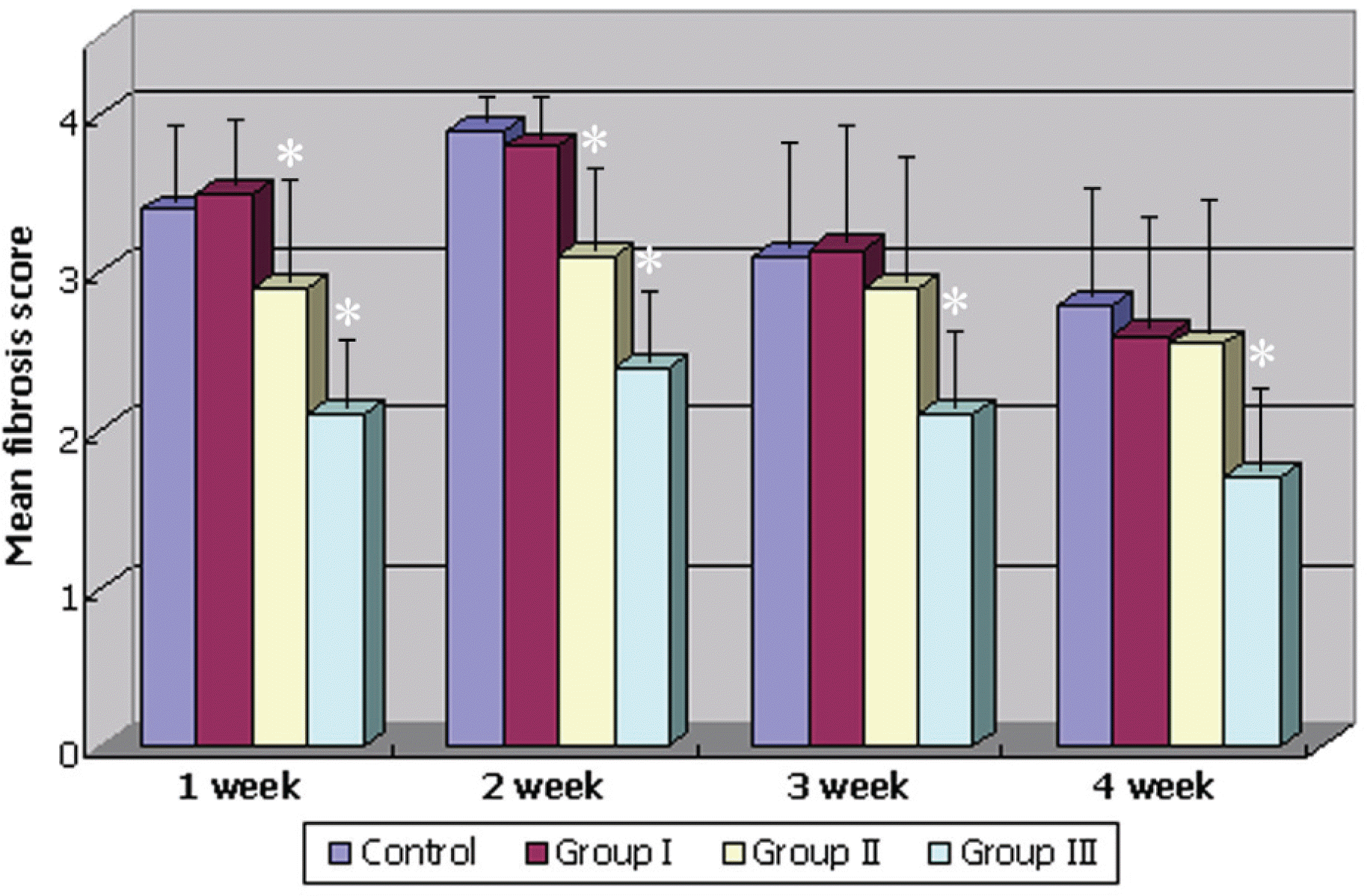 | Figure 2.Graphs of the overall opacity rate according to the fibrosis score. Group I=treated with TGF-β inhibitor 10 μg; Group II=treated with TGF-β inhibitor 25 μg; Group III=treated with TGF-β inhibitor 50 μg (Asterisk: clinically significant compared with the control group, p<0.05). |
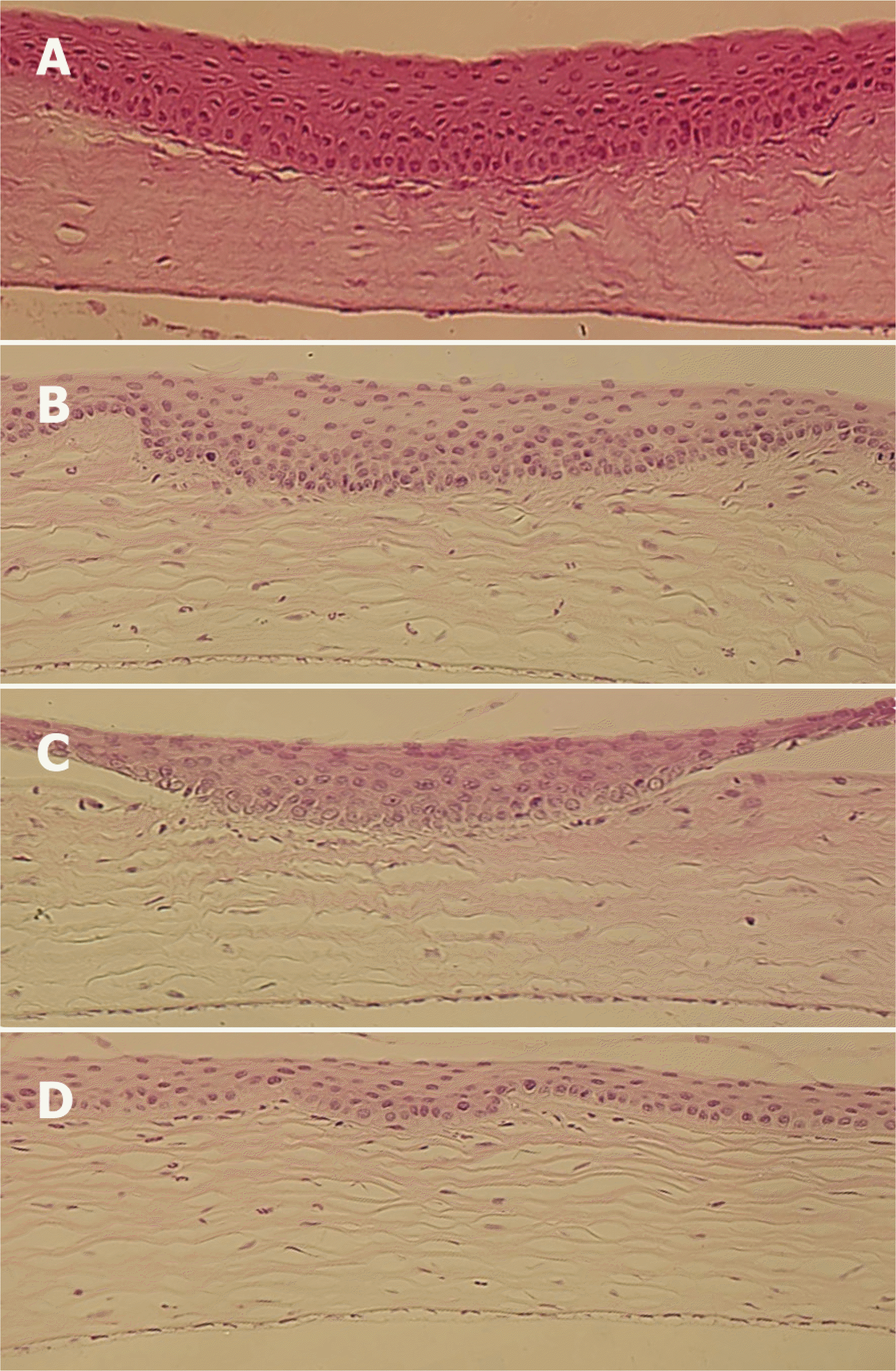 | Figure 3.Light microscopic findings of the corneas at 2 weeks after corneal laceration (H&E stain, ×400). (A) Control group (B) Group I (treated with TGF-β inhibitor 10 μg) (C) Group II (treated with TGF-β inhibitor 25 μg) (D) Group III (treated with TGF-β inhibitor 50 μg). The increasing density of keratocytes in the stormal layer was observed in the control group and group I compared with groups II and III. |
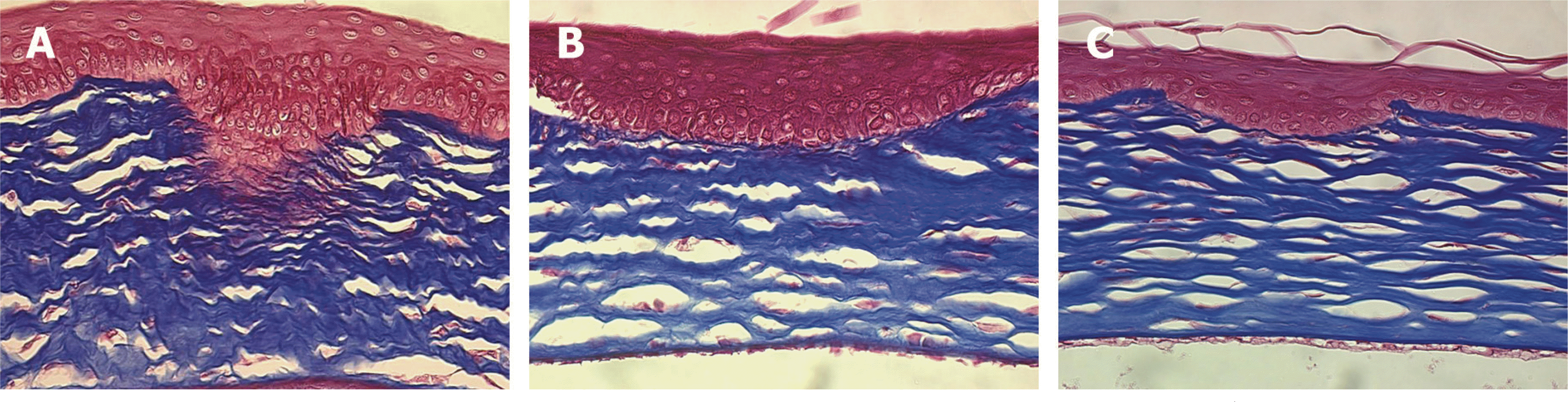 | Figure 4.Light microscopic findings of the corneas at 2 weeks after corneal laceration (Masson trichrome stain, ×400). (A) Control group (B) Group II (treated with TGF-β inhibitor 25 μg) (C) Group III (treated with TGF-β inhibitor 50 μg). The irregular arrangement of the collagen lamella in the stromal layer was observed. In group III, however, the irregularity of the collagen lamella was less and thickness of the epithelial plug was thinner than in the other groups. |
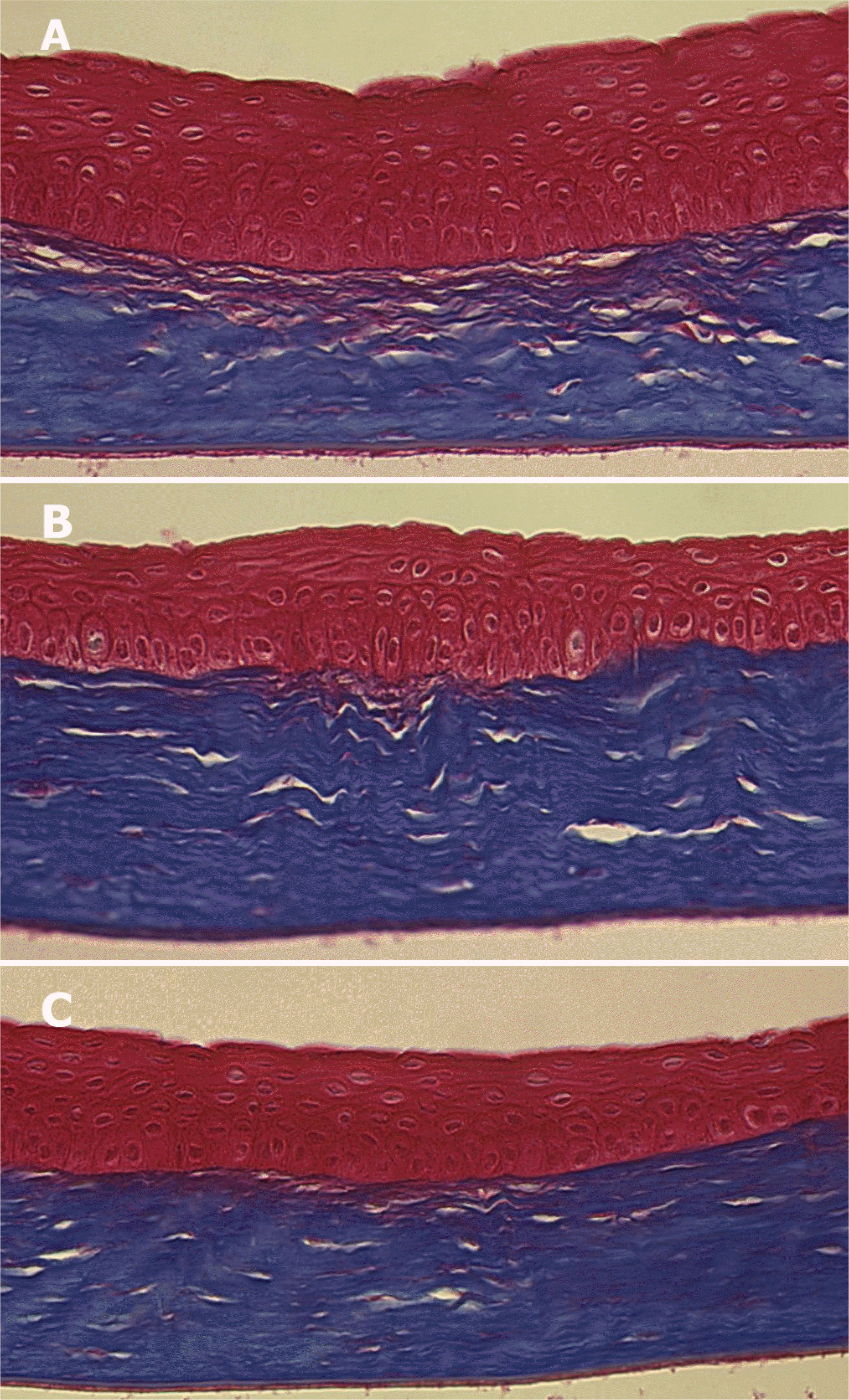 | Figure 5.Light microscopic findings of the corneas at 4 weeks after corneal laceration (Masson trichrome stain, ×400). (A) Control group (B) Group II (treated with TGF-β inhibitor 25 μg) (C) Group III (treated with TGF-β inhibitor 50 μg). The irregularity of the regenerated collagen lamella was significantly less in group III than in the other groups. |
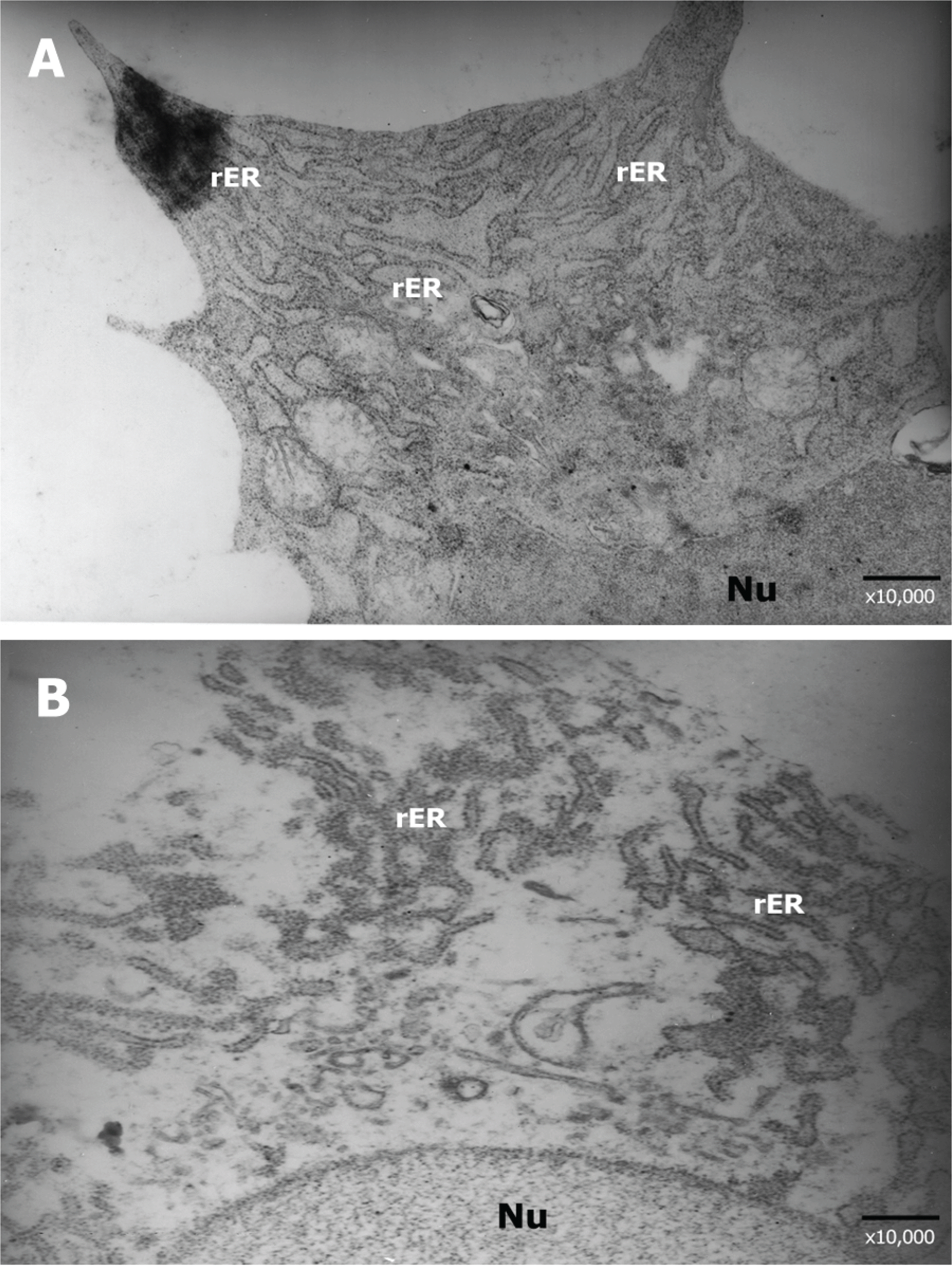 | Figure 6.Transmission electron photomicrographs of corneal keratocytes at 4 weeks after corneal laceration (×10,000). (A) Control group, (B) Group III (treated with TGF-β inhibitor 50 μg). The well- developed rough endoplasmic reticulum (rER) was observed in keratocytes within the corneal laceration wound site. However, the keratocytes in group III showed decreased number and density of the rough endoplasmic reticulum than in the control group. |
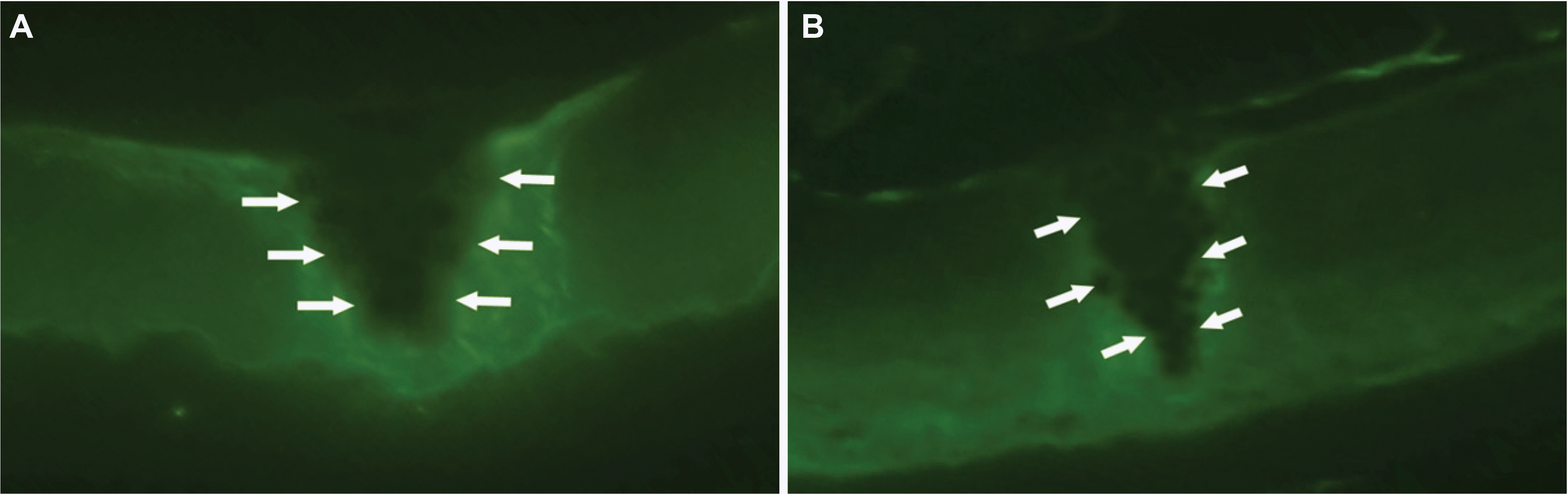 | Figure 7.The fluorescence photomicrographs of the corneas at 4 weeks after corneal laceration (DTAF stain, ×200). (A) Control group (B) Group III (treated with TGF-β inhibitor 50 μg). The area of the regenerated corneal stroma is thinner in group III than control group; bright green fluorescent area represents original stromal bed (arrow) and dark non-fluorescent area represents regenerated corneal stroma. |
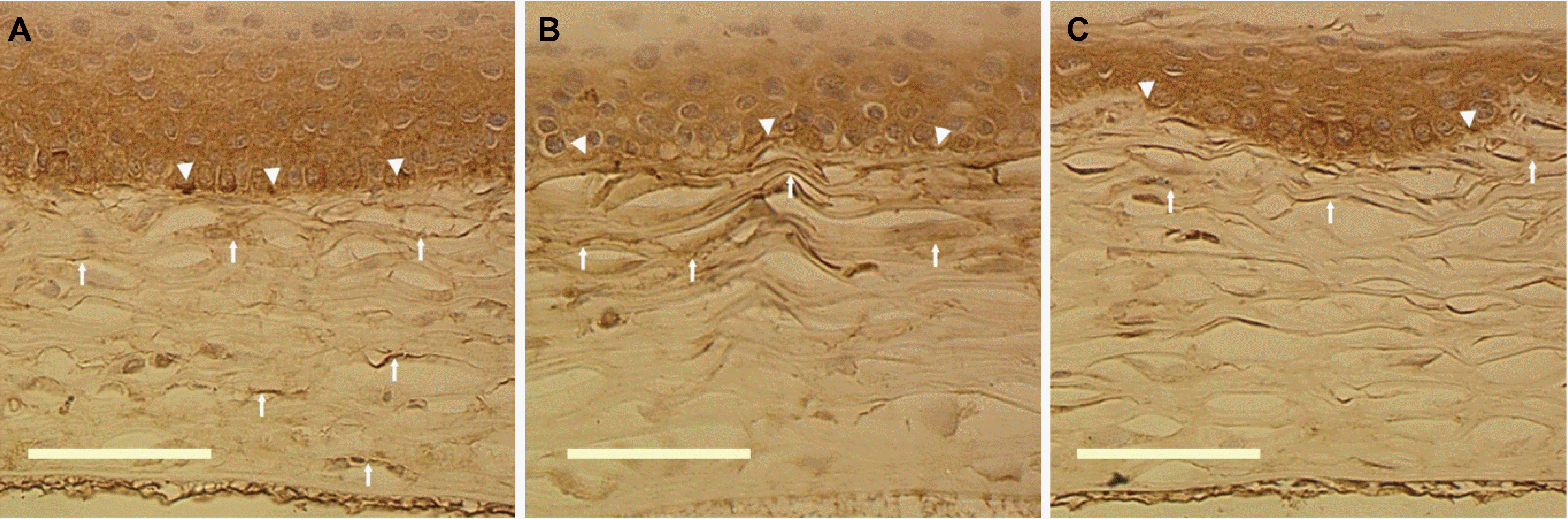 | Figure 8.The immunocytochemical stainings of fibronectin at 2 weeks after corneal laceration (×400, Scale bar=50 μm). (A) Control group (B) Group II (treated with TGF-β inhibitor 25 μg) (C) Group III (treated with TGF-β inhibitor 50 μg). The immunoreactivity of fibronectin was observed at subepithelial (arrowhead) and regenerated stromal lamellar area in all groups. However, the expression of fibronectin decreased according to increasing concentration of TGF-β inhibitor. |
 | Figure 9.The immunocytochemical stainings of alpha-smooth muscle actin at 2 weeks after corneal laceration (×400, Scale bar=50 μm). (A) Corneal semithin section of control group (B) Corneal semithin section of group III (treated with TGF-β inhibitor 50 μg). (C) Semithin section of the iris in group III (Asterisk: blood vessels; arrow: iris muscle). The immunoreactivity of alpha-smooth muscle actin (arrowhead) was observed in stromal keratocytes within healing area. However, the number of immunoreactive keratocytes in group III was less than in control group. No significant differences of immunoreactivity between keratocytes (arrowhead) and iris muscle (arrow). |
Table 1.
Comparison of the keratocyte number in the stroma around corneal laceration site
| 2 weeks | 4 weeks | |||||
|---|---|---|---|---|---|---|
| Anterior stroma* | Posterior stroma* | Total† | Anterior stroma* P | Posterior stroma* | Total† | |
| Control | 17.7±2.5 | 9.4±1.9 | 27.2±4.0 | 25.1±4.6 | 13.6±2.1 | 38.8±6.8 |
| Group I | 17.9±5.8 | 8.6±2.5 | 26.4±7.9 | 24.3±6.5 | 12.9±2.4 | 37.2±9.0 |
| Group II | 13.2±3.4‡ | 8.0±0.8 | 21.2±4.1‡ | 22.9±3.9 | 13.2±1.0 | 36.0±4.7 |
| Group III | 11.6±1.4‡ | 5.1±1.3‡ | 16.8±2.7‡ | 18.4±3.1‡ | 8.1±2.2‡ | 26.6±5.5‡ |
Table 2.
Mean thickness of the regenerated corneal stroma at 4 weeks after corneal laceration
| Anterior stroma* | Posterior stroma† | Mean thickness‡ | |
|---|---|---|---|
| Control | 85.6±24.5 μm | 61.4±29.0 μm | 78.3±32.1 μm |
| Group I | 80.1±38.8 μm | 62.5±30.4 μm | 74.0±43.9 μm |
| Group II | 63.9±31.2 μm | 54.8±20.8 μm | 61.2±33.6 μm |
| Group III | 45.1±17.4 μm§ | 39.3±12.3 μm§ | 42.5±19.0 μm§ |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download