Abstract
Purpose
To compare histopathological and apoptotic changes of ophthalmoscopically similar subthreshold laser burns made by a low power-long duration (LD) and a high power-short duration (SD) subthreshold laser treatment.
Methods
Ophthalmoscopically invisible subthreshold laser burns with a 3.0 mm spot size were made using an 810 nm diode laser on the rabbit retina. Lasers were applied for 60 seconds in the LD group, and 1 second in the SD group. Laser power was adjusted to achieve ophthalmoscopically invisible burns just below the threshold. The rabbits were sacrificed at 6, 12, 24, and 72 hours, 1, 2, and 4 weeks after laser treatment. The eyes were processed for light microscopic examination using hematoxylin and eosin (H&E), toluidine blue, and TdT-dUTP terminal nick-end labeling (TUNEL) staining. Eyes were also processed for electron microscopic examination.
Results
The changes in the retina were different between the two groups. The LD group showed abundant TUNEL positive cells in all the retinal layers at 6 hours after laser treatment, and distinct histological changes in the outer nuclear layer. Conversely, in the SD group, apoptosis did not occur and histological alteration in the outer nuclear layer was minimal.
Go to : 
References
1. Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 1991; 109:1220–31.
2. Macular Photocoagulation Study Group. Subfoveal neovascular lesions in age-related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 1991; 109:1232–41.
3. Oosterhuis JA, Journee-de Korver HG, Kakebeeke-Kemme HM, Bleeker JC. Transpupillary thermotherapy in choroidal melanomas. Arch Ophthalmol. 1995; 113:315–21.

4. Shields CL, Shields JA, Cater J, et al. Transpupillary thermotherapy for choroidal melanoma: tumor control and visual results in 100 consecutive cases. Ophthalmology. 1998; 105:581–90.
5. Kim US, Yu SY, Kim SW, Kwak HW. Five cases of transpupillary thermotherapy for intraocular tumors. J Korean Ophthalmol Soc. 2003; 44:2942–49.
6. Oh JW, Yun IH. Transpupillary thermotherapy in circumscribed choroidal hemangioma. J Korean Ophthalmol Soc. 2003; 44:992–7.
7. Reichel E, Berrocal AM, Ip M, et al. Transpupillary thermotherapy of occult subfoveal choroidal neovascularization in patients with age-related macular degeneration. Ophthalmology. 1999; 106:1908–14.

8. Newsom RS, McAlister JC, Saeed M, McHugh JD. Transpupillary thermotherapy (TTT) for the treatment of choroidal neovascularization. Br J Ophthalmol. 2001; 85:173–8.
9. Algvere PV, Libert C, Seregard S. Transpupillary thermotherapy of occult CNV with no or minimally classic CNV in age-related macular degeneration. Semin Ophthalmol. 2001; 16:90–6.

10. Morimura Y, Okada AA, Hayashi A, et al. Histological effect and protein expression in subthreshold transpupillary thermotherapy in rabbit eyes. Arch Ophthalmol. 2004; 122:1510–5.
11. She H, Li X, Yu W. Subthreshold transpupillary thermotherapy of the retina and experimental choroidal neovascularization in a rat model. Graefes Arch Clin Exp Ophthalmol. 2006; 244:1143–51.

12. Welch AJ, Wissler EH, Priebe LA. Significance of blood flow in calculation of temperature in laser irradiated tissue. IEEE Trans Biomed Eng. 1980; 27:164–6.
13. Welch AJ, Polhamus GD. Measurement and prediction of thermal injury in the retina of the rhesus monkey. IEEE Trans Biomed Eng. 1984; 31:633–43.

14. Parver LM, Auker C, Carpenter DO. Choroidal blood flow as a heat dissipating mechanism in the macula. Am J Ophthalmol. 1980; 89:641–6.

16. Mainster MA, White TJ, Tips JH, Wilson PW. Retinal- temperature increases produced by intense light sources. J Opt Soc Am. 1970; 60:264–70.
17. Vassiliadis A. Laser sources and ocular effects. Francis A, L'esperance JR, editors. Ophthalmic lasers. 3rd ed.St. Louis: The C.V. Mosby company;1989. 1:chap. 2.
18. Mainster MA. Decreasing retinal photocoagulation damage: principles and techniques. Semin Ophthalmol. 1999; 14:200–9.

19. Desmettre T, Maurage CA, Mordon S. Heat shock protein hyperexpression on chorioretinal layers after transpupillary thermot-herapy. Invest Ophthalmol Vis Sci. 2001; 42:2976–80.
20. Desmettre T, Maurage CA, Mordon S. Transpupillary thermotherapy (TTT) with short duration laser exposure induce heat shock protein (HSP) hyperexpression on choroidoretinal layers. Lasers Surg Med. 2003; 33:102–7.
21. Mainster MA, Reichel E. Transpupillary thermotherapy for age-related macular degeneration: long-pulse photocoagulation, apoptosis, and heat shock proteins. Ophthalmic Surg Lasers. 2000; 31:359–73.

22. Samali A, Orrenius S. Heat shock proteins: regulators of stress response and apoptosis. Cell Stress Chaperones. 1998; 3:228–36.

23. Mainster MA. Wavelength selection in macular photocoagulation. Tissue optics, thermal effects, and laser systems. Ophthalmology. 1986; 93:952–8.
Go to : 
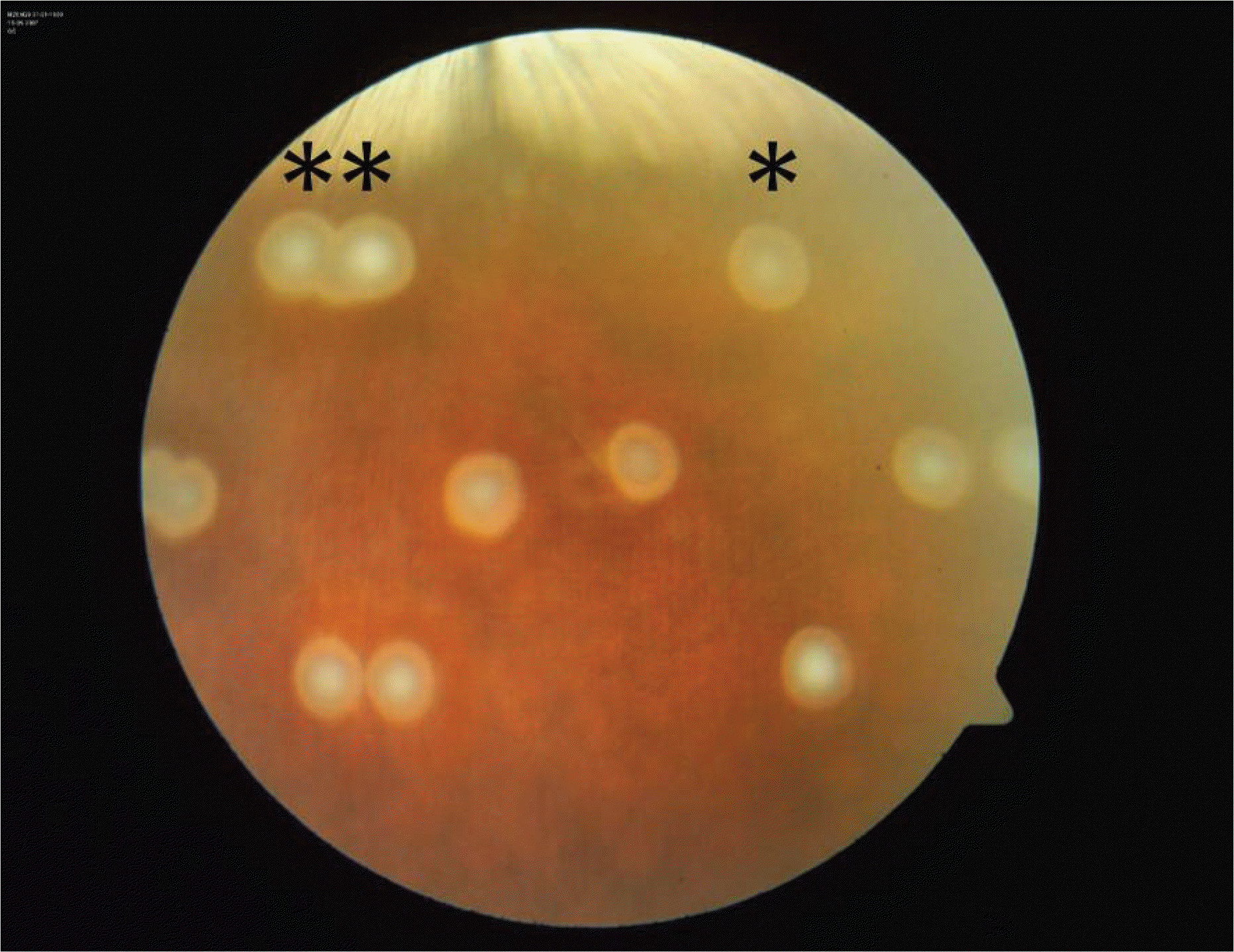 | Figure 1.Fundus photograph of marker laser burns: Single laser (*) was applied as a superior and inferior marker for the long duration subthreshold laser treatment (LD) and two juxtaposed lasers (**) for the short duration subthreshold laser treatment (SD). Subthres-hold lasers of 3.0 mm in spot size had been applied for 60 seconds in the LD group, and for 1 second for SD group within the marker burns. |
 | Figure 2.H&E stained histological findings of the long duration (LD, A and C) and short duration (SD, B and D) subthreshold laser group. ×400. (A) LD at 12 hours, (B) SD at 12 hours, (C) LD at 4 weeks, and (D) SD at 4 weeks after the laser treatment. Retina became swollen soon after the subthreshold laser treatment in both groups. Vacuole formation in the cytoplasm of inner nuclear layer is more evident in the LD group (A and B). Altered photoreceptor inner and outer segments, and intracytoplasmic vacuolization in the retinal pigment epithelium are noted. Choroid did not show any remarkable alteration except for mild stromal edema. Retinal thickness has decreased with time in the both group, while reduction of cellularity was more marked in the LD group. |
 | Figure 3.TUNEL staining of the long duration (LD, A and C) and short duration (SD, B and D) subthreshold laser group, ×400. (A) LD at 6 hours, (B) SD at 6 hours, (C) LD at 24 hours, and (D) SD at 24 hours after the laser treatment. Numerous TUNEL-positive cells stained brown are observed in GCL, INL and ONL at 6 hours after long duration subthreshold laser treatment. They decreased rapidly with time. GCL=ganglion cell layer; INL=inner nuclear layer; ONL=outer nuclear layer. |
 | Figure 4.Transmission electron micrograph of LD group. (A) Six hours after the laser. Focal eruption of retinal pigment epithelium with severe cytoplasmic alteration is observed. (Original magnification ×2,000; bar indicates 2 µm) (B) At 6 hours. Damaged ONL with pyknosis and altered cytoplasm of the cells are noted. (×2,500; bar, 2 µm) (C) At 12 hours. Vacuolizations in the cytoplasm of retinal pigment epithelial cell and disrupted photo-receptor outer segments are observed. Several phagosomes dislodged from the outer segment are scattered in the cytoplasm. (×2,500; bar, 2 µm) (D) One week after LD laser. Damaged photoreceptor inner and outer segments are closely associated with retinal pigment epithelial cells that had lost their apical microvillus. Vacuoles are still present in the RPE cytoplasm. (×2,500; bar indicates 2 µm) |
 | Figure 5.Transmission electron micrograph of SD group. (A) Six hours after the laser. Apical microvilli were destroyed and numerous vacuoles are observed in the cytoplasm of the RPE. (original magnification ×6,500; bar indicates 1 µm) (B) At 6 hours. (×3,000; bar, 2 µm) Pyknosis and abnormal cytoplasm with heterochromatin condensation is observed but is less significant than LD group. (C) At 12 hours. Deformed erythrocyte and obstructed capillary lumen beneath the well preserved Bruch membrane is seen. Photoreceptor outer segments are disrupted and several phagosomes engulfed in the cytoplasm of the retinal pigment epithelium are noted. (×3,000; bar, 2 µm) (D) At 1 week. The number of photoreceptor outer segment decreased. Note the altered apical microvilli and vacuoles in the cytoplasm of retinal pigment epithelial cell. (×2,500; bar, 2 µm) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download