Abstract
Purpose
To report the anti-inflammatory effect and best-corrected visual acuity (BCVA) response after oral glucosamine (Glucosamine Sulfate 750®, General Nutrition Companies, Inc.) and aspirin (Aspirin protect®, Bayer) therapy in patients with uveitis.
Methods
Twenty-seven patients (43 eyes) with uveitis, not easily managed with conventional therapy, were treated with oral glucosamine 750 mg and aspirin 100 mg daily, and underwent BCVA testing and slit-lamp examination of anterior chamber inflammation. Changes in the dose of previously-used oral steroids and immunosuppressants were recorded at baseline, every 2 weeks for the first 2 months, and once every month thereafter.
Results
The mean follow-up period was 15.0±3.69 weeks and inflammation started to improve within 4.6 weeks. The change in visual acuity was not statistically significant, but grade of inflammation decreased by as much as 1.02±1.28, significantly (p<0.01) after treatment. In 12 patients (19 eyes, 44.2%) the dosage of systemic steroids and immunosuppressants could be Reduced. 7 patients (10 eyes, 23.3%) experienced aggravation of uveitis and the dosage of glucosamine and aspirin was increased.
Conclusions
Concurrent oral administration of glucosamine and aspirin could not completely inhibit the recurrence of inflammation nor control it, but consistent use of these drugs may help to reduce inflammation without serious side effects. A longer prospective control study with larger sample size is required to further evaluation of the efficacy of the medication.
Go to : 
References
1. Dick AD, Azim M, Forrester JV. Immunosuppressive therapy for chronic uveitis: optimizing therapy with steroids and cyclosporine A. Br J Ophthalmol. 1997; 81:1107–12.
2. De Vries J, Baarsma GS, Zaal MJ, et al. Cyclosporin in the treatment of severe chronic idiopathic uveitis. Br J Ophthalmol. 1990; 74:344–9.

3. Kuk JH, Jung WJ, Jo GH, et al. Production of N-acetyl-beta-D- glucosamine from chitin by Aeromonas sp. GJ-18 crude enzyme. Appl Microbiol Biotechnol. 2005; 68:384–9.
4. Alvarez-Soria MA, Largo R, Calvo E, et al. Differential anticatabolic profile of glucosamine sulfate versus other anti- osteoarthritic drugs on human osteoarthritic chondrocytes and synovial fibroblasts in culture. Osteoarthritis Cartilage. 2005; 13:153.
5. Reginster JY, Bruyere O, Neuprez A. Current role of glucosamine in the treatment of osteoarthritis. Rheumatology. 2007; 46:731–35.

6. Botting RM. Inhibitors of cyclooxygenases: mechanisms, selec-tivity and uses. J Physiol Pharmacol. 2006; 57:113–24.
7. Chen JT, Chen CH, Horng CT, et al. Glucosamine sulfate inhibits proinflammatory cytokine-induced ICAM-1 production in human conjunctival cells in vitro. J Ocular Pharmacol Ther. 2006; 22:402–16.
8. Chen JT, Liang JB, Chou CL, et al. Glucosamine sulfate inhibits TNF-α and IFN-γ induced production of ICAM-1 in human retinal pigment epithelial cells in vitro. Invest Ophthalmol Vis Sci. 2006; 47:664–72.
9. Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of Uveitis Nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005; 140:509–16.
10. Forrester JV, Mc Menamin PG. Immunopathogenic mechanisms in intraocular inflammation. Chem Immunol. 1999; 73:159–85.

13. Largo R, Alvasrez-Soria MA, Diez-Ortego I, et al. Glucosamine inhibits IL-1beta-induced NF kappa B activation in human osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2003; 11:290–8.
14. Wang XC, Jobin C, Allen JB, et al. Suppression of NF- kappaB dependent proinflammatory gene expression in human RPE cells by a proteasome inhibitor. Invest Ophthalmol Vis Sci. 1999; 40:477–86.
15. Platts KE, Benson MT, Rennie IG, et al. Cytokine modulation of adhesion molecule expression on human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1995; 36:2262–9.
16. Abraham c, Griffith J, Miller J. The dependence for leukocyte function-associated antigen-1/ICAM-1 interactions in T cell activation cannot be overcome by expression of high density TCR ligand. J Immunol. 1999; 162:4399–405.
17. Pham T, Cornea A, Blick KE, et al. Oral glucosamine in doses used to treat osteoarthritis worsens insulin resistance. Am J Med Sci. 2007; 333:333–9.

18. Ossendza RA, Grandval P, Chinoune F, et al. Acute cholestatic hepatitis due to glucosamine forte. Gastroenterol Clin Biol. 2007; 31:449–50.
Go to : 
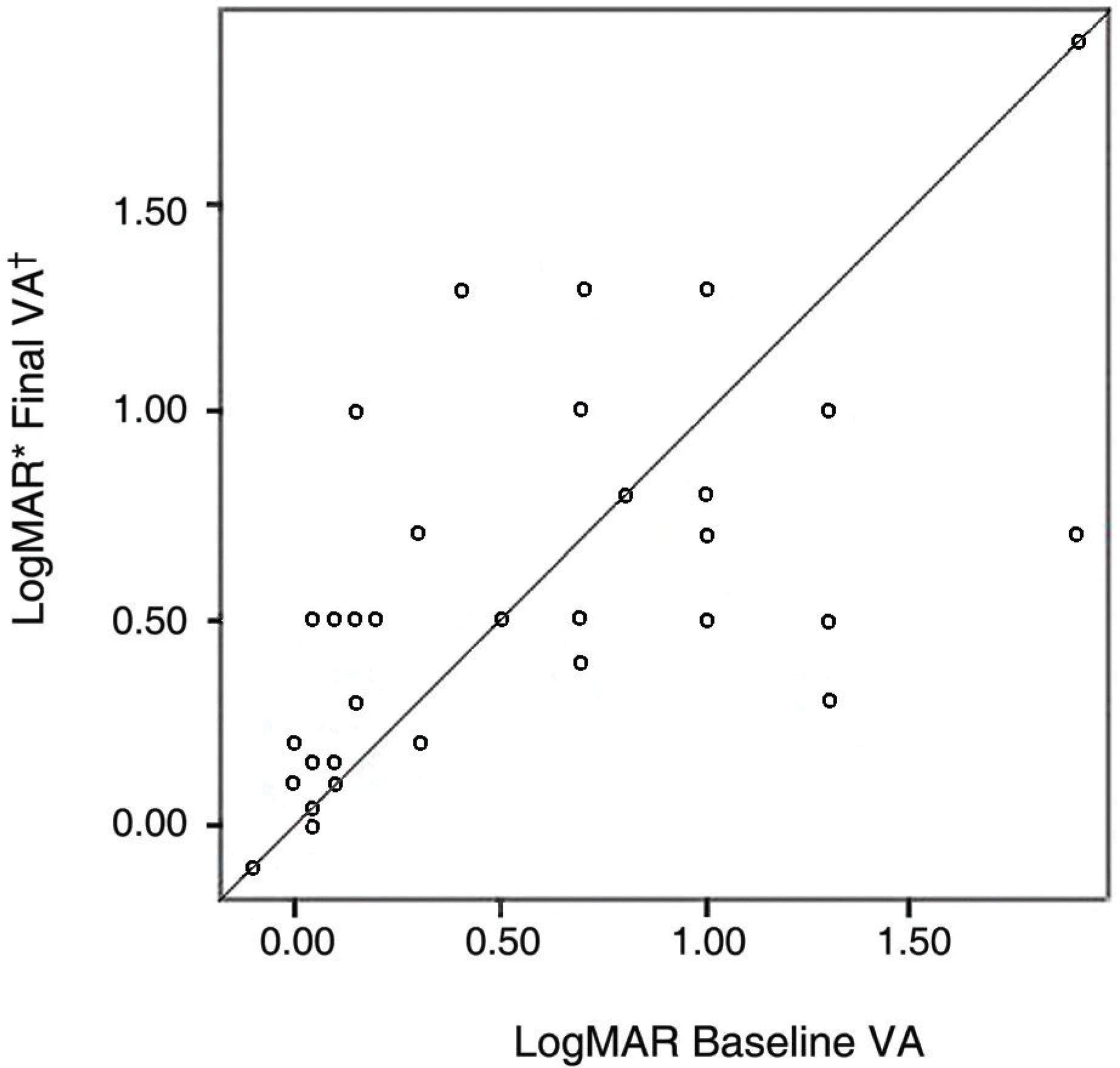 | Figure 1.The relationship between baseline visual acuity and final visual acuity after glucosamine & aspirin treatment in uveitis. * MAR=minimal angle of resolution; † VA=visual acuity. |
Table 1.
Baseline Characteristics
Table 2.
Initiation of Glucosamine sulfate and Aspirin (G&A) Therapy
| Reason for starting G&A | No. of patients (%) | No. of eyes (%) |
|---|---|---|
| Recent activation* | ||
| Aggravated chamber cell reaction† | 12 (44.4%) | 21 (48.8%) |
| Newly developed PSII‡ | 4 (14.8%) | 4 (9.3%) |
| Failed use of prednisolone due to side effects | 5 (18.5%) | 7 (16.3%) |
| Poor control of inflammation despite sufficient immunosuppression | 6 (22.2%) | 11 (25.6%) |
Table 3.
Contribution of G & A to previous treatment
Table 4.
Outcomes of G & A therapy for control of inflammation
| Outcomes | No. of patients (%) | No. of eyes (%) |
|---|---|---|
| Reduction of systemic steroid dose | 7 (25.9%) | 12 (27.9%) |
| Reduction of concomitant immunosuppressants | 5 (18.5%) | 7 (16.3%) |
| Cessation of using steroid or immunosuppressants | 2 (7.4%) | 12 (27.9%) |
| Improved activity* | 16 (59.3%) | 26 (60.5%) |
| Remission† | 15 (55.6%) | 24 (55.8%) |
| Increment of steroid dose due to worsening activity‡ | 7 (25.9%) | 10 (23.3%) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download