Abstract
Purpose
To evaluate the long-term results of intravitreal bevacizumab injection for macular edema (ME) due to retinal vein obstruction (RVO) and diabetic retinopathy (DR).
Methods
The objects of study were patients with decreased visual acuity due to ME with RVO and DR for whom intravitreal injections of 1.25 mg (0.05 ml) bevacizumab were repeated three times with an interval of six weeks and who were available for a follow-up period of more than 12 months. The patients underwent additional bevacizumab injections if ME increased as assessed by optical coherence tomography (OCT). Best-corrected visual acuity (BCVA) and central macular thickness (CMT) were measured at baseline and follow-up visits.
Results
There were 16 patients with RVO and 18 patients with DR. In the RVO group, the mean length of follow-up was 12.4±1.1 months, the mean baseline BCVA was 0.75±0.32 and the final BCVA was 0.42±0.25, a difference that was statistically significant (p<0.05). The mean CMT at baseline was 588.5±301.0 µm and this decreased to a mean of 191.8±112.0 µm at the end of the follow-up period (p<0.05). In the DR group, the mean length of follow-up was 15.4±3.2 months, the mean baseline BCVA was 0.63±0.33 and the final BCVA was 0.61±0.37, a difference that was not statistically significant (p>0.05). The mean CMT at baseline was 462.0±195.0 µm and decreased to a mean of 282.2±177.3 µm at the end of the follow-up period (p<0.05).
References
1. Ryan SJ. Retina. Dick SB, Jampol LM, Haller JA, editors. Macular edema. 3rd ed.St. Louis: The C.V. Mosby Co;2001. 2:chap. 57.
2. Takahashi MK, Hikichi T, Akiba J, et al. Role of the vitreous and macular edema in branch retinal vein occlusion. Ophthalmic Surg Lasers. 1997; 28:294–9.
4. Gutman FA, Zegarra H. Macular edema secondary to occlusion of the retinal vein. Surv Ophthalmol. 1984; 28:462–70.
5. Finkelstein D. Ischemic macular edema: recognition and favorable natural history in branch vein occlusion. Arch Ophthalmol. 1992; 110:1427–34.

6. Finkelstein D. Laser treatment of macular edema resulting from branch vein occlusion. Semin Ophthalmol. 1994; 9:23–8.

7. The Branch Vein Occlusion Study Group. Argon laser photo-coagulation for macular edema in branch vein occlusion. Am J Ophthalmol. 1984; 98:271–82.
8. The Central Vein Occlusion Study Group. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. The Central Vein Occlusion Study Group M report. Ophthalmology. 1995; 102:1425–33.
9. Early Treatment Diabetic Retinopathy Study Group. Photocoagulation for diabetic macular edema. ETDRS report no. 1 Arch Ophthalmol. 1985; 103:1796–806.
10. Early Treatment Diabetic Retinopathy Study Group. Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report no.19 Arch Ophthalmol. 1995; 113:1144–55.
11. Hwang JH, Cho YW. The Efficiency of Vitrectomy for Diabetic Macular Edema. J Korean Ophthalmol Soc. 2003; 44:1079–84.
12. Jonas JB, Hayler JK, Panda-Jonas S. Intravitreal injection of crystalline cortisone as adjunctive treatment of proliferative vitreoretinopathy. Br J Ophthalmol. 2000; 84:1064–7.

13. Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002; 109:920–7.

14. Greenberg PB, Martidis A, Rogers AH, et al. Intravitreal triamcinolone acetonide for macular oedema due to central retinal vein occlusion. Br J Ophthalmol. 2002; 86:247–8.

15. Antcliff RJ, Spalton DJ, Stanford MR, et al. Intravitreal triamcinolone for uveitic cystoid macular edema: an optical coherence tomography study. Ophthalmology. 2001; 108:765–72.

16. Jonas JB, Sofker A. Intraocular injection of crystalline cortisone as adjunctive treatment of diabetic macular edema. Am J Ophthalmol. 2001; 132:425–7.

17. Park CH, Jaffe GJ, Fekrat S. Intravitreal triamcinolone acetonide in eyes with cystoid macular edema associated with central retinal vein occlusion. Am J Ophthalmol. 2003; 136:419–25.

18. Jonas JB, Hayler JK, Sofker A, Panda-Jonas S. Intravitreal injection of crystalline cortisone as adjunctive treatment of proliferative diabetic retinopathy. Am J Ophthalmol. 2001; 131:468–71.

19. Tolentino MJ Miller JW, Gragoudas ES, et al. Intravitreal injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996; 103:1820–8.
20. Funatsu H Yamashita H, Nakamura S, et al. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2006; 113:294–301.

21. Noma H, Funatsu H, Yamasaki M, et al. Pathogenesis of macular edema with branched retinal vein obstruction and intraocular levels of vascular endothelial growth factor and interleukin-6. Am J Ophthalmol. 2005; 140:256–61.
22. Brooks HL Jr, Caballero S Jr, Newell CK, et al. Vitreous levels of vascular endothelial growth factor and stromalderived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch Ophthalmol. 2004; 122:1801–7.
23. Itakura H, Akiyama H, Hagimura N, et al. Triamcinolone acetonide suppresses interleukin-1 beta mediated increase in vascular endothelial growth factor expression in cultured rat Muller cells. Graefes Arch Clin Exp Ophthalmol. 2006; 244:226–31.
24. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005; 36:331–5.

25. Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, et al. Pan-American Collaborative Retina Study Group (PACORES). Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007; 114:743–50.
26. Wu L, Arevalo JF, Roca JA, et al. Pan-American Collaborative Retina Study Group (PACORES). Comparison of two doses of intravitreal bevacizumab (Avastin) for treatment of macular edema secondary to branch retinal vein occlusion: results from the Pan-American Collaborative Retina Study Group at 6 months of follow-up. Retina. 2008; 28:212–9.
27. Aiello LP, Bursell SE, Clermont A, et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997; 46:1473–80.

28. Vinores SA, Youssri AO, Luna JD, et al. Upregulation of vascularendothelial growth factor in ischemic and nonischemic human and experimental retinal disease. Histol Histopathol. 1997; 12:99–109.
29. Pe'er J, Folberg R, Itin A, et al. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology. 1998; 105:412–6.
30. Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol. 1999; 237:130.

31. Aiello LP, Northrup JM, Keyt BA, et al. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol. 1995; 113:1538–44.

32. Pournaras JA, Nguyen C, Vaudaux JD, et al. Treatment of central retinal vein occlusion-related macular edema with intravitreal bevacizumab (avastin): preliminary results. Klin Monatsbl Augenheilkd. 2008; 225:397–400.

33. Stahl A, Agostini H, Hansen LL, Feltgen N. Bevacizumab in retinal vein occlusion-results of a prospective case series. Graefes Arch Clin Exp Ophthalmol. 2007; 245:1429–36.

34. Costa RA, Jorge R, Calucci D, et al. Intravitreal bevacizumab (avastin) for central and hemicentral retinal vein occlusions: IBeVO study. Retina. 2007; 27:141–9.
35. Höh AE, Schaal KB, Scheuerle A, et al. OCT-guided reinjection of 2.5 mg bevacizumab for treating macular edema due to retinal vein occlusion. Ophthalmologe. 2008; 105:1121–6.
36. Badalà F. The treatment of branch retinal vein occlusion with bevacizumab. Curr Opin Ophthalmol. 2008; 19:234–8.

37. Rabena MD, Pieramici DJ, Castellarin AA, et al. Intravitreal bevacizumab (Avastin) in the treatment of macular edema secondary to branch retinal vein occlusion. Retina. 2007; 27:419–25.

38. Gündüz K, Bakri SJ. Intravitreal bevacizumab for macular oedema secondary to branch retinal vein occlusion. Eye. 2008; 22:1168–71.

39. Duff IF, Fall HF, Linman JW. Anticoagulant therapy in occlusive vascular disease of retina. Arch Ophthalmol. 1951; 46:601–17.
40. Michel RG, Gass JD. The natural course of retinal branch vein obstruction. Trans Am Acad Ophthalmol Otolaryngol. 1974; 78:166–77.
41. Zhang Y, Fortune B, Atchaneeyasakul LO, et al. Natural history and histology in a rat model of laser-induced photothrombotic retinal vein occlusion. Curr Eye Res. 2008; 33:365–76.

42. Ozkiriş A. Intravitreal bevacizumab (Avastin) for primary treatment of diabetic macular oedema. Eye. 2008; 25:[Epub ahead of print].

43. Haritoglou C, Kook D, Neubauer A, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006; 26:999–1005.

44. Byeon SH, Kwon YA, Oh HS, et al. Short-term results of intravitreal bevacizumab for macular edema with retinal vein obstruction and diabetic macular edema. J Ocul Pharmacol Ther. 2007; 23:387–94.

45. Fraser-Bell S, Kaines A, Hykin PG. Update on treatments for diabetic macular edema. Curr Opin Ophthalmol. 2008; 19:185–9.

46. Shimura M, Nakazawa T, Yasuda K, et al. Comparative therapy evaluation of intravitreal bevacizumab and triamcinolone acetonide on persistent diffuse diabetic macular edema. Am J Ophthalmol. 2008; 145:854–61.

47. Ozdemir H, Karacorlu M, Karacorlu SA. Regression of serous macular detachment after intravitreal triamcinolone acetonide in patients with diabetic macular edema. Am J Ophthalmol. 2005; 140:251–5.

48. Meleth AD, Agron E, Chan CC, et al. Serum inflammatory markers in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005; 46:4295–301.

49. Wu L, Martínez-Castellanos MA, Quiroz-Mercado H, et al. Twelve-month safety of intravitreal injections of bevacizumab: results of the Pan-American Collaborative Retina Study Group (PACORES). Graefes Arch Clin Exp Ophthalmol. 2008; 246:81–7.
Figure 1.
Changes in best-corrected visual acuity (BCVA, logMAR) after intravitreal bevacizumab. RVO (retinal vein occlusion) group shows general improvement of visual acuity after 1 st, 2 nd injection of bevacizumab, and visual acuity seems to be maintained from the time of the 3 rd injection to 12 months after injection. The mean baseline BCVA was 0.75±0.32 and the final BCVA was 0.42±0.25, a difference that was statistically significant (p<0.05). DR (diabetic retinopathy) group also shows improvement of visual acuity after injection of bevacizumab but the degree is small and visual acuity appears to decrease from 18 weeks after injection. The mean baseline BCVA was 0.63±0.33 and the final BCVA was 0.61±0.37, a difference that was not statistically significant (p>0.05).
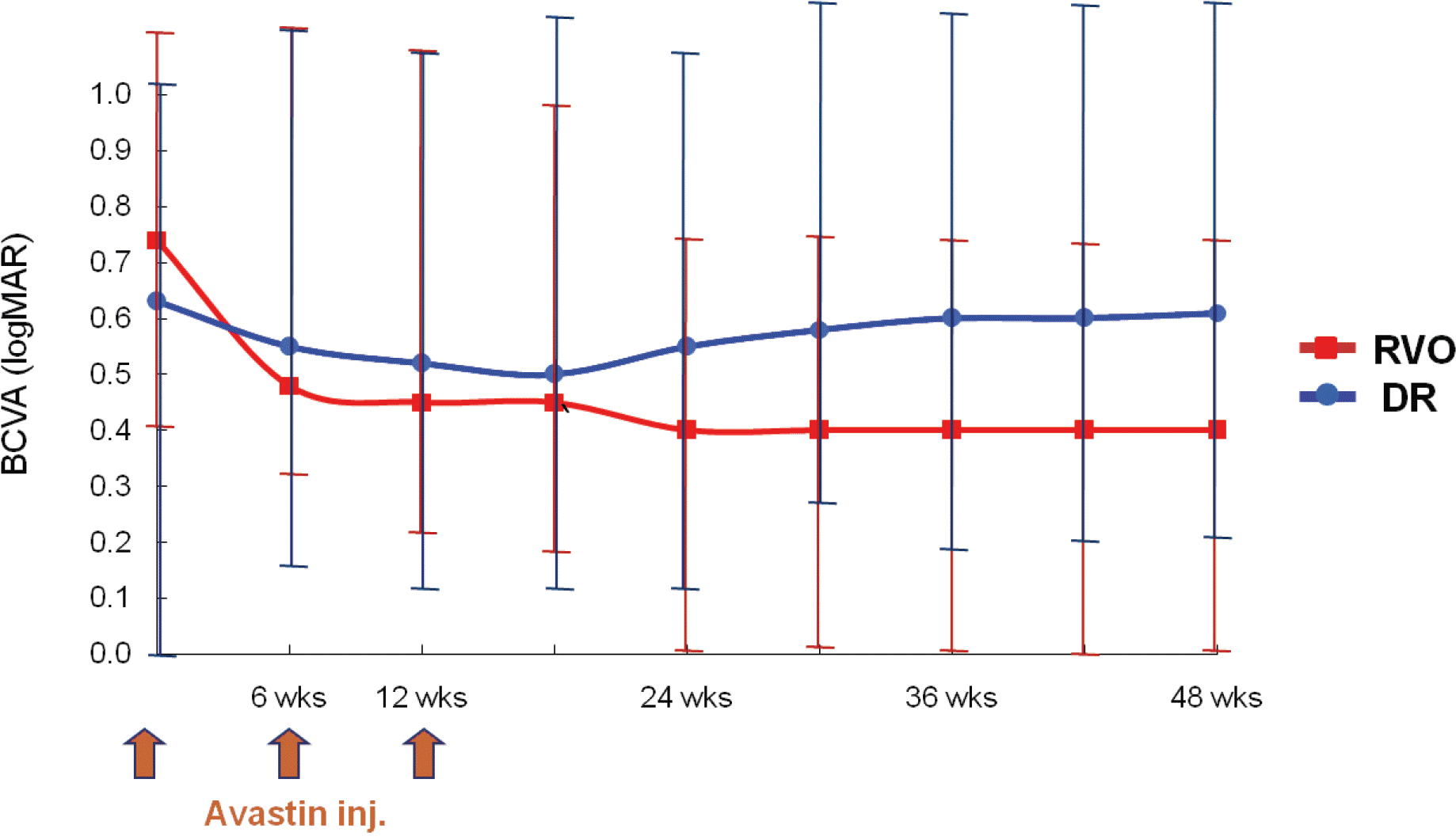
Figure 2.
Final best-corrected visual acuity (BCVA) analysis after intravitreal bevacizumab. RVO (retinal vein occlusion) group demonstrated that 10(62.5%) eyes improved ≥ 2 lines of BCVA, 5(31.3%) eyes remained stable, 1(6.3%) eye decreased ≥ 2 lines of BCVA with Snellen chart. DR (diabetic retinopathy) group demonstrated that 5(27.8%) eyes improved ≥2 lines of BCVA, 6(33.3%) eyes remained stable, 7(38.8%) eyes decreased ≥ 2 lines of BCVA with Snellen chart.
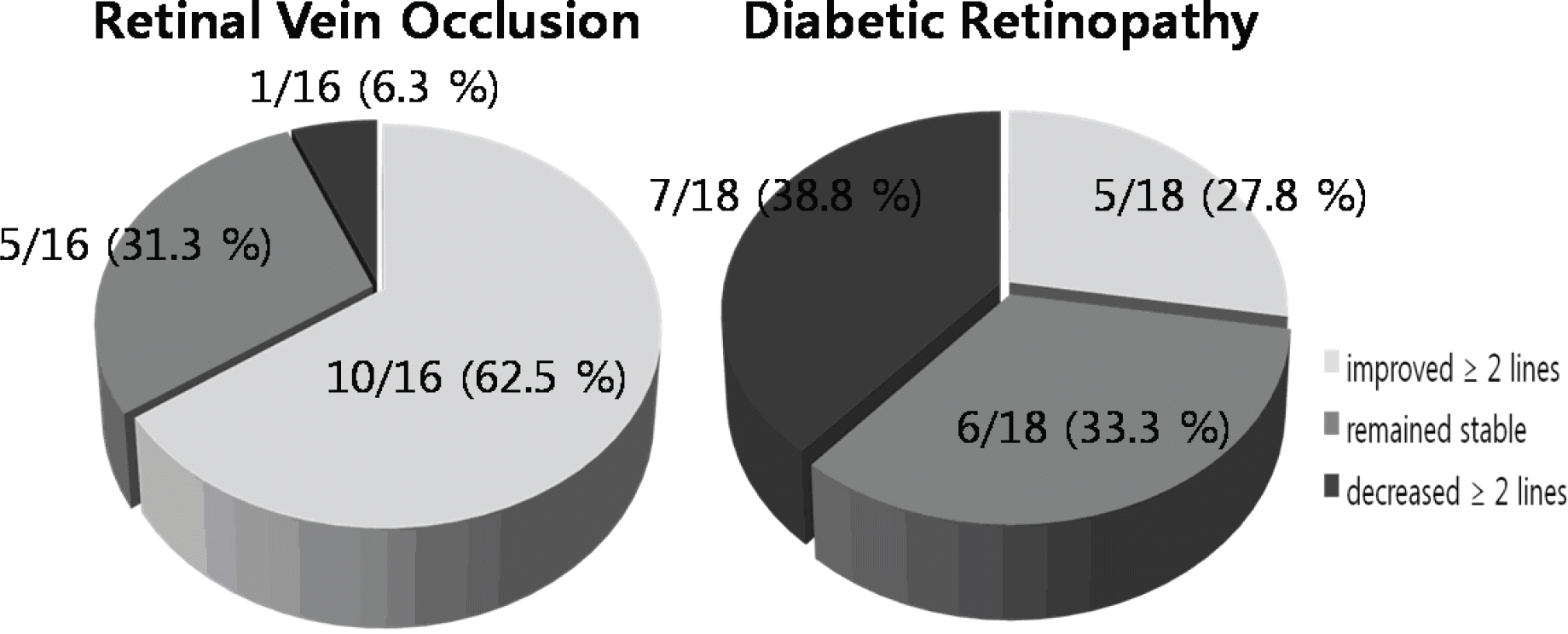
Figure 3.
Changes in central macular thickness after intravitreal bevacizumab. RVO (retinal vein occlusion) groupshows general decrease of macular edema after injection of bevacizumab and seems to be maintained from the time of the 3rd injection to 12 months after injection. DR (diabetic retinopathy) group also shows improvement of macular edema after injection of Bevacizumab but the degree is small than RVO group. Both RVO and DR groups showed statistically significant decrease of macular thickness than baseline (RVO: 588.5±301.0 µm 191.8±112.0 µm, DR: 462.0±195.0 µm 282.2±177.3 µm, p<0.05).
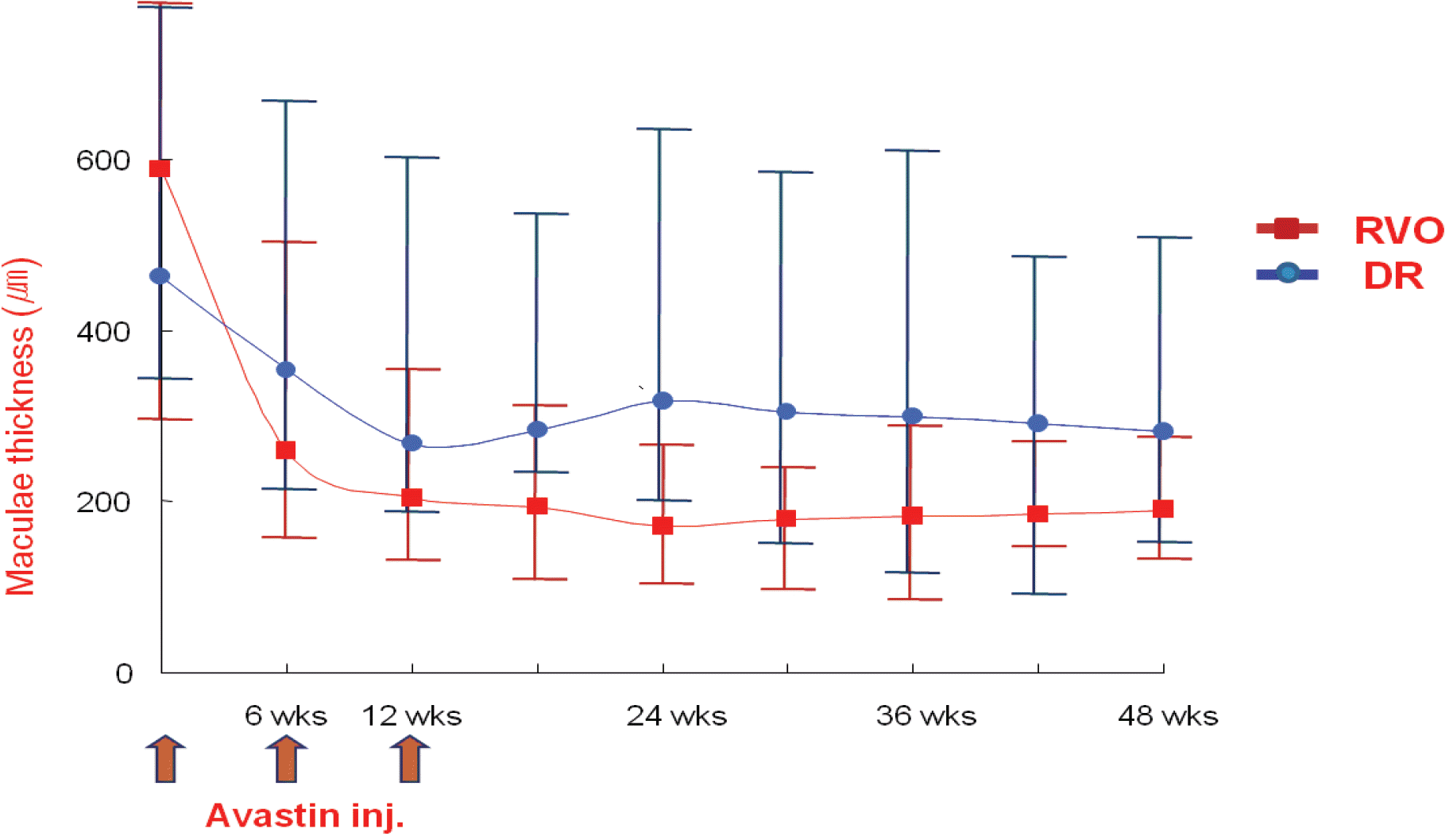
Figure 4.
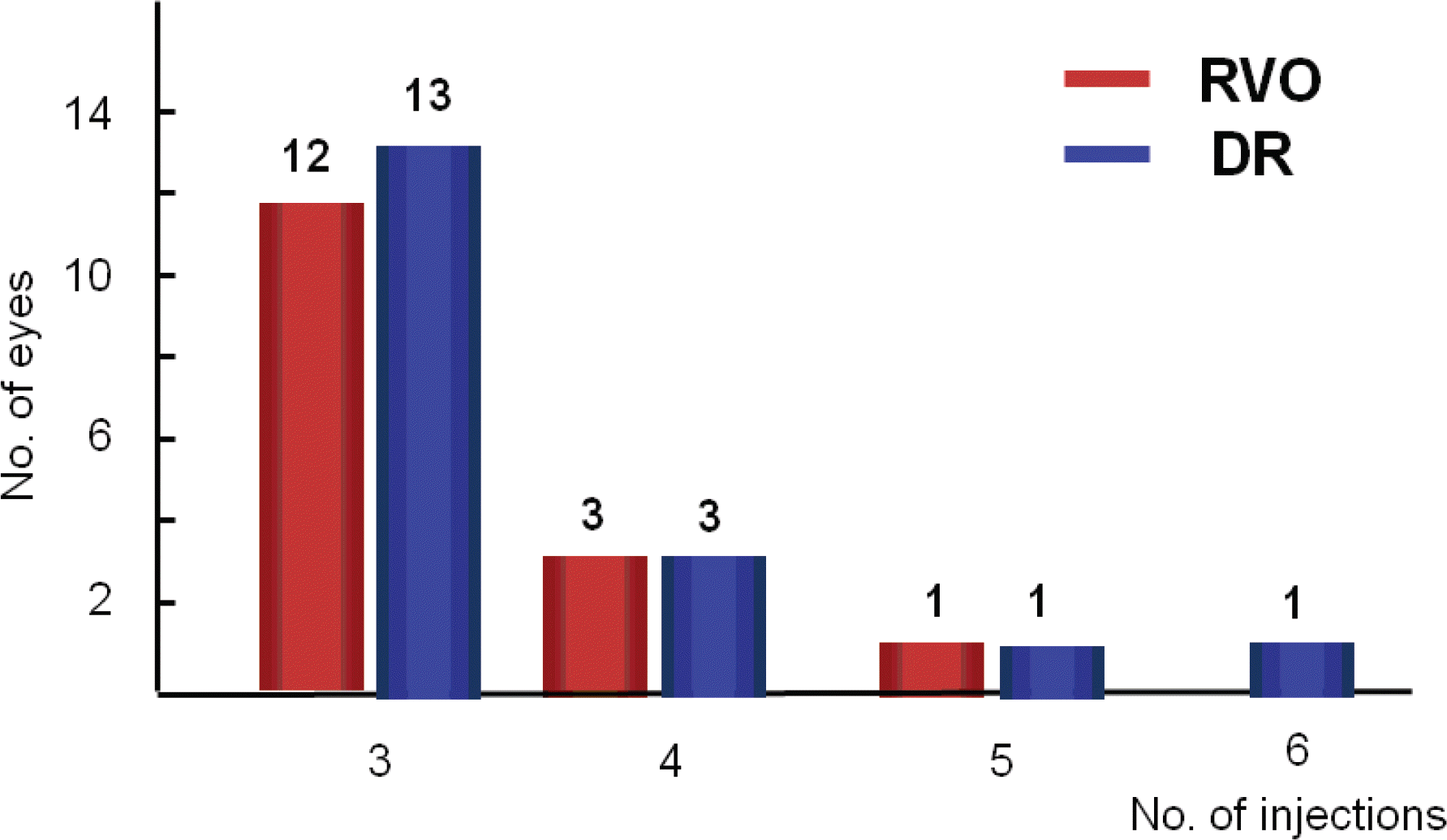
Number of intravitreal bevacizumab injections in retinal vein occlusion and diabetic retinopathy for 12-month follow-up.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download