Abstract
Purpose
To investigate the effects and prognostic factors related to intravitreal injection of bevacizumab on patients with subfoveal choroidal neovascularization secondary to age-related macular degeneration.
Methods
The medical records of patients who received 3 consecutive intravitreal injections of bevacizumab (1.25 mg/0.05 ml, 6 weeks interval) for subfoveal choroidal neovascularization secondary to age-related macular degeneration and followed up for more than 12 months were reviewed (a total of 31 eyes; male, 20; mean age, 72.3±7.5 years). Baseline best corrected visual acuity, foveal thickness, and total macular volume were compared with those after 1, 4, and 12 months. The therapeutic effects were investigated with regard to factors such as age, sex, initial visual acuity, lesion size, subtypes of choroidal neovascularization, pigment epithelial detachment, submacular hemorrhage, and previous history of photodynamic therapy.
Results
Initial visual acuity (logMAR), foveal thickness, and total macular volume were 0.74±0.49, 320±88 μm and 9.50±2.99 mm3, respectively. Visual acuity improved to 0.68±0.61 (p=0.012), and foveal thickness and total macular volume decreased to 218±69 μm and 6.32±0.71 mm3 (p<0.001), respectively, at 12 months. Visual improvement was achieved less often in patients who were 75 years or older and who had lesions 3 disc areas or greater and relatively good initial vision at 12 months.
Conclusions
Intravitreal bevacizumab injection has beneficial effects for patients with subfoveal choroidal neovascularization secondary to age-related macular degeneration with regard to function and anatomy. However, it should be noted that visual improvement may be limited in older patients with larger lesions and good initial vision.
Go to : 
References
1. Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004; 122:564–72.
2. Klein R, Klein BEK, Knudtson MD, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the Multi-ethnic Study of Atherosclerosis. Arch Ophthalmol. 2006; 113:373–80.

3. Macular Photocoagulation Study Group. Laser photocoacu-lation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials. Arch Ophthalmol. 1993; 111:1200–9.
4. Treatment of Age-Related Macular Degeneration with Photo-dynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin. One-year results of 2 randomized clinical trials-TAP report. Arch Ophthalmol. 1999; 117:1329–45.
5. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997; 18:4–25.

6. Shen WY, Yu MJ, Barry CJ, et al. Expression of cell adhesion and vascular endothelial growth factor in experimental choroidal neovascularization in the rat. Br J Ophthalmol. 1998; 82:1063–71.
7. Krzystolik MG, Afshari MA, Adamis AP, et al. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol. 2002; 120:338–46.

8. Quiram PA, Hassan TS, Williams GA. Treatment of naïve lesions in neovascular age-related macular degeneration with pegaptanib. Retina. 2007; 27:851–6.

9. Kaiser PK, Blodi BA, Shapiro H, et al. Angiographic and optical, coherence tomographic results of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmol. 2007; 114:1868–75.

10. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus Verteporfin for Neovascular Age-Related Macular Degeneration. N Engl J Med. 2006; 355:1432–44.

11. Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004; 3:391–400.

12. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006; 113:363–72.

13. Rich RM, Rosenfeld PJ, Puliafito CA, et al. Short-term safety and efficacy of intravitreal bevacizumab (Avastin) for neo-vascular age-related macular degeneration. Retina. 2006; 26:495–511.

14. Spaide RF, Laud K, Fine HF, et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006; 26:383–90.

15. Hijikata K, Masuda K. Visual prognosis in Behet's disease: effects of cyclophosphamide and colchicines. Jpn J Ophthalmol. 1978; 22:506–19.
16. Kaiser PK Treatment of Age-Related Macular Degeneration with Photodynamic Theraphy (TAP) Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: 5-year results of two randomized clinical trials with an open-label extension: TAP report no. 8. Graefes Arch Clin Exp Ophthalmol. 2006; 244:1132–42.
17. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss; AREDS report no. 8. Arch Ophthalmol. 2001; 119:1417–36.
18. Kaiser PK, Brown DM, Zhang K, et al. Ranibizumab for Predo-minantly Classic Neovascular Age-related Macular Degeneration: Subgroup Analysis of First-year ANCHOR Result. Am J Ophthalmol. 2007; 144:850–7.
19. Gibran SK, Khan K, Junqkim S, Cleary PE. Optical coherence tomographic pattern may predict visual outcome after intravitreal triamcinolone for diabetic macular edema. Ophthalmology. 2007; 114:890–4.

20. Boyer DS, Antoszyk AN, Awh CC, et al. MARINA Study Group. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007; 114:246–52.
21. Bakri SJ, Nickel J, Yoganathan P, Beer PM. Photodynamic therapy for choroidal neovascularization associated with submacular hemorrhage in age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2006; 37:278–83.

22. Heier JS, Boyer DS, Ciulla TA, et al. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration: year 1 results of FOCUS Study. Arch Ophthalmol. 2006; 124:1532–42.
23. Yuzawa M, Hagita K, Egawa T, et al. Macular lesions predisposing to senile disciform macular degeneration. Jpn J Ophthalmol. 1991; 35:87–95.
24. Chan WM, Lai TY, Tano Y, et al. Photodynamic therapy in macular diseases of asian populations: when East meets West. Jpn J Ophthalmol. 2006; 50:161–9.

25. Lim JI, Kwok A, Wilson DK. Symptomatic age-related macular degeneration in Asian patients. Retina. 1998; 18:435–8.

26. Japanese Age-Related Macular Degeneration Trial (JAT) Study Group. Japanese age-related macular degeneration trial: 1-year results of photodynamic therapy with verteporfin in Japanese patients with subfoveal choroidal neovascularization secondary to age-related macular degeneration. Am J Ophthalmol. 2003; 136:1049–61.
27. Yu HG, Kang SW, Nam WH, et al. Photodynamic therapy for choroidal neovascularization secondary to age-related macular degeneration. J Korean Ophthalmol Soc. 2007; 48:789–98.
28. Bressler NM Treatment of Age-related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photo-dynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-TAP Report 2. Arch Ophthalmol. 2001; 119:198–207.
29. Bashshur ZF, Haddad ZA, Schakal A, et al. Intravitreal bevacizumab for treatment of neovascular age-related macular degeneration: a one-year prospective study. Am J Ophthalmol. 2008; 145:249–56.

30. Ignoffo RJ. Overview of vevacizumab: a new cancer therapeutic strategy targetingvascular endothelial growth factor. Am J Health Syst Pharm. 2004; 61:21–6.
31. Fung AE, Rosenfeld PJ, Reichel E. The International Intravitreal Avastin Safety Survey: using the Internet to assess drug safety worldwide. Br J Ophthalmol. 2006; 90:1344–9.
Go to : 
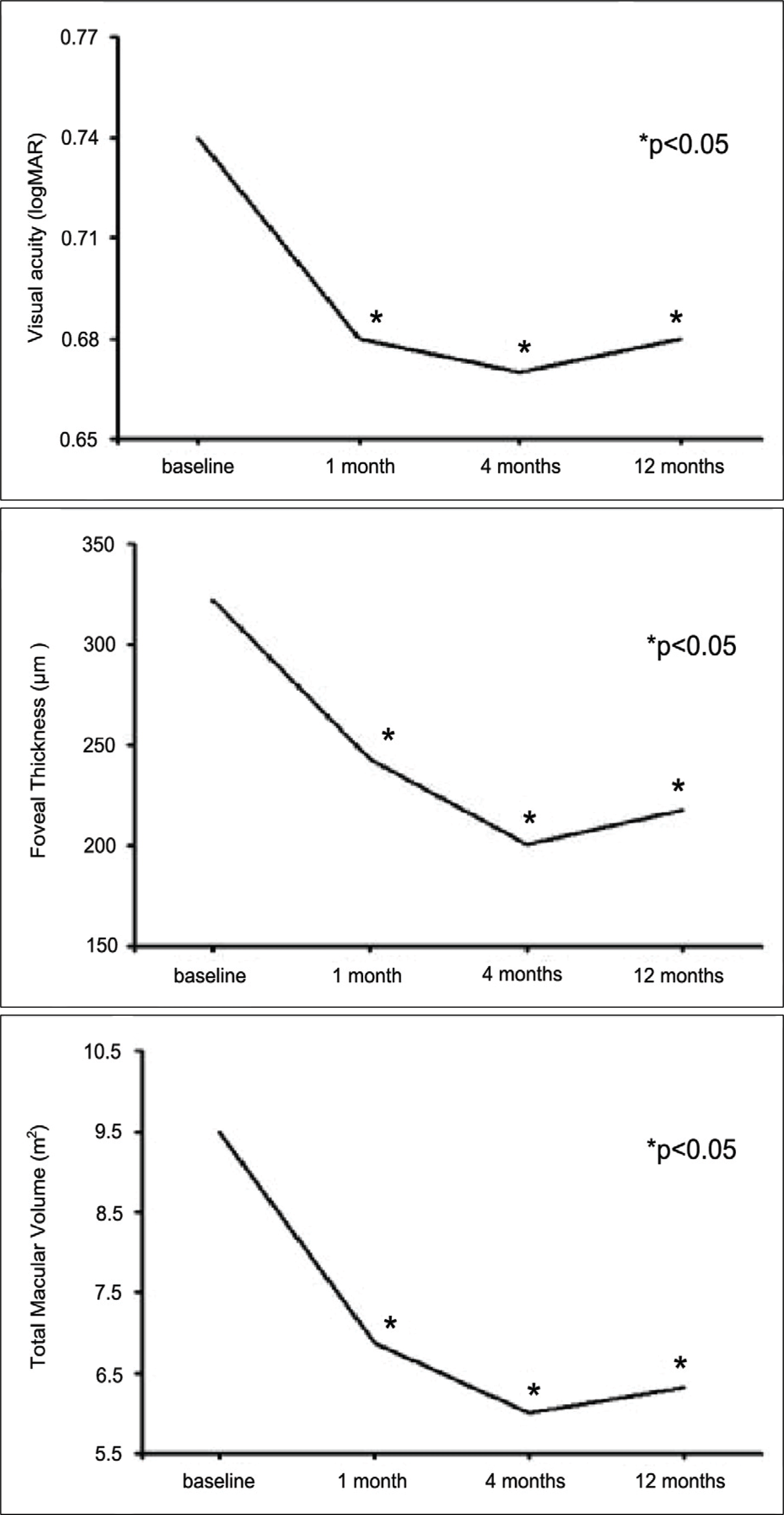 | Figure 1.Changes in visual acuity (logMAR), foveal thickness, total macular volume during follow-up. |
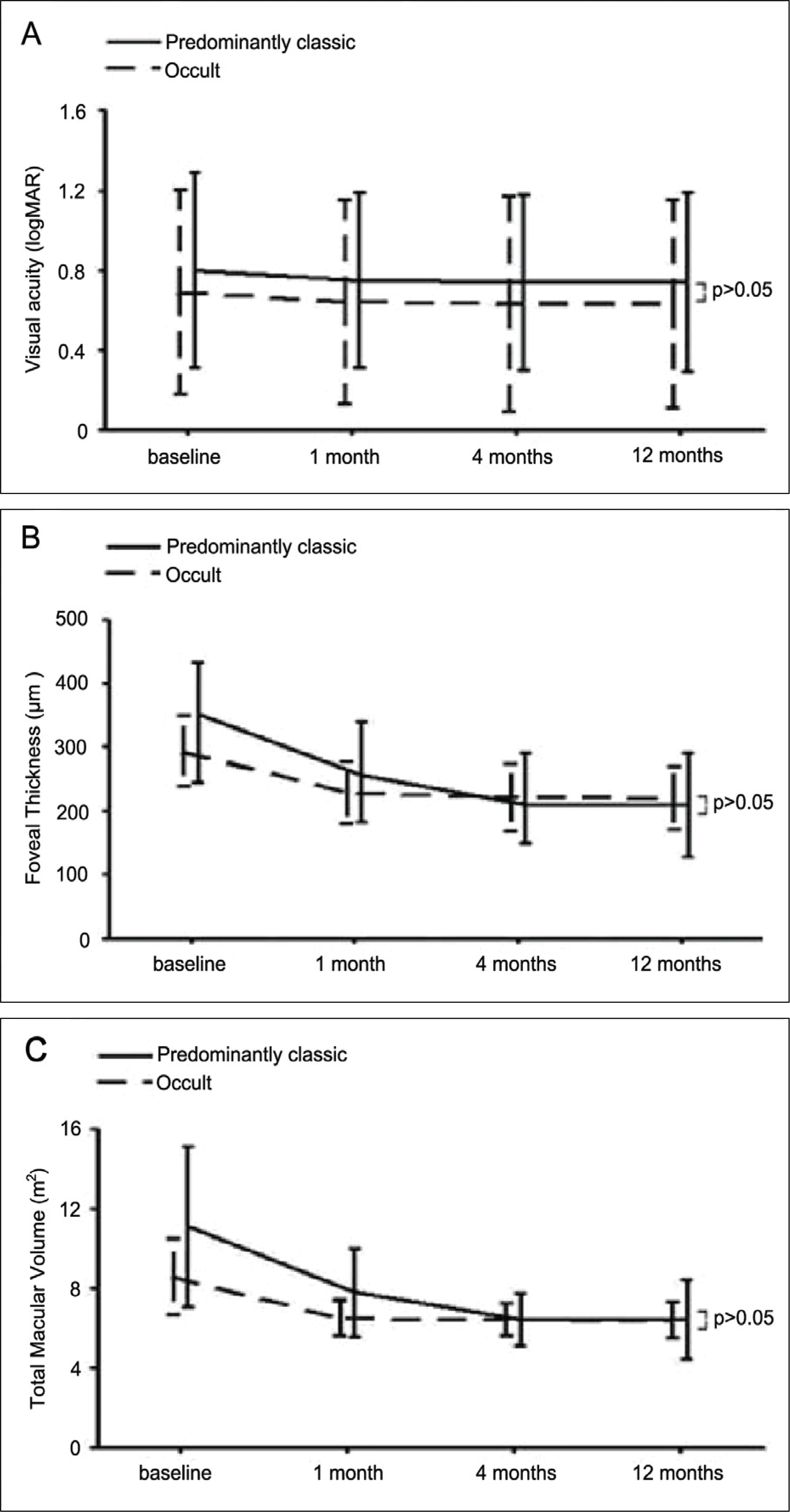 | Figure 2.Comparision of visual acuity, foveal thickness, total macular volume between predominantly classic and occult subfoveal choroidal neovascularization secondary to AMD during the 12-month follow-up. No statistically significant differences were shown between two groups. |
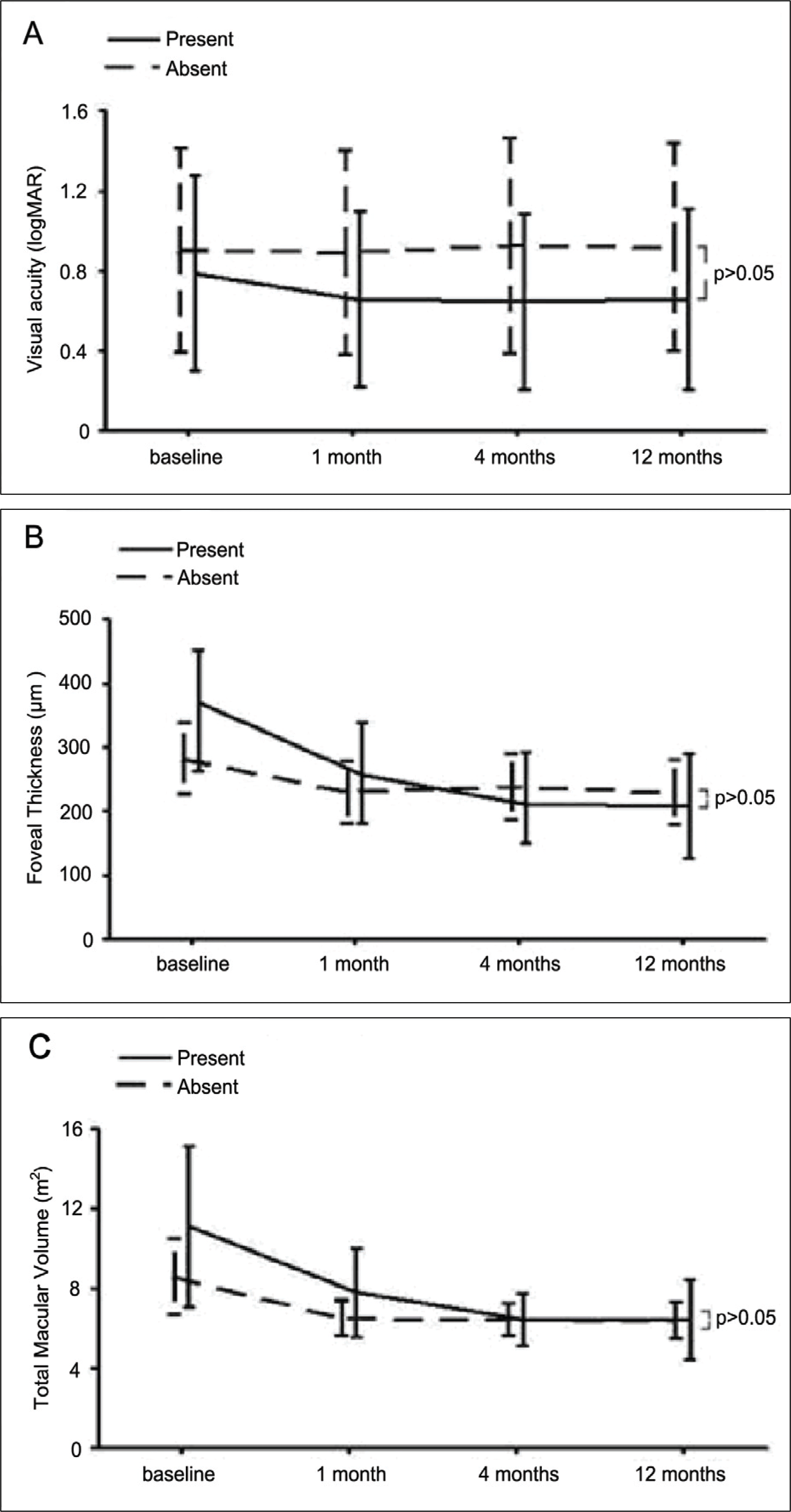 | Figure 3.Comparision of visual acuity, foveal thickness, total macular volume depending on presence of PED during the 12-month follow-up. No statistically significant differences were shown between two groups. |
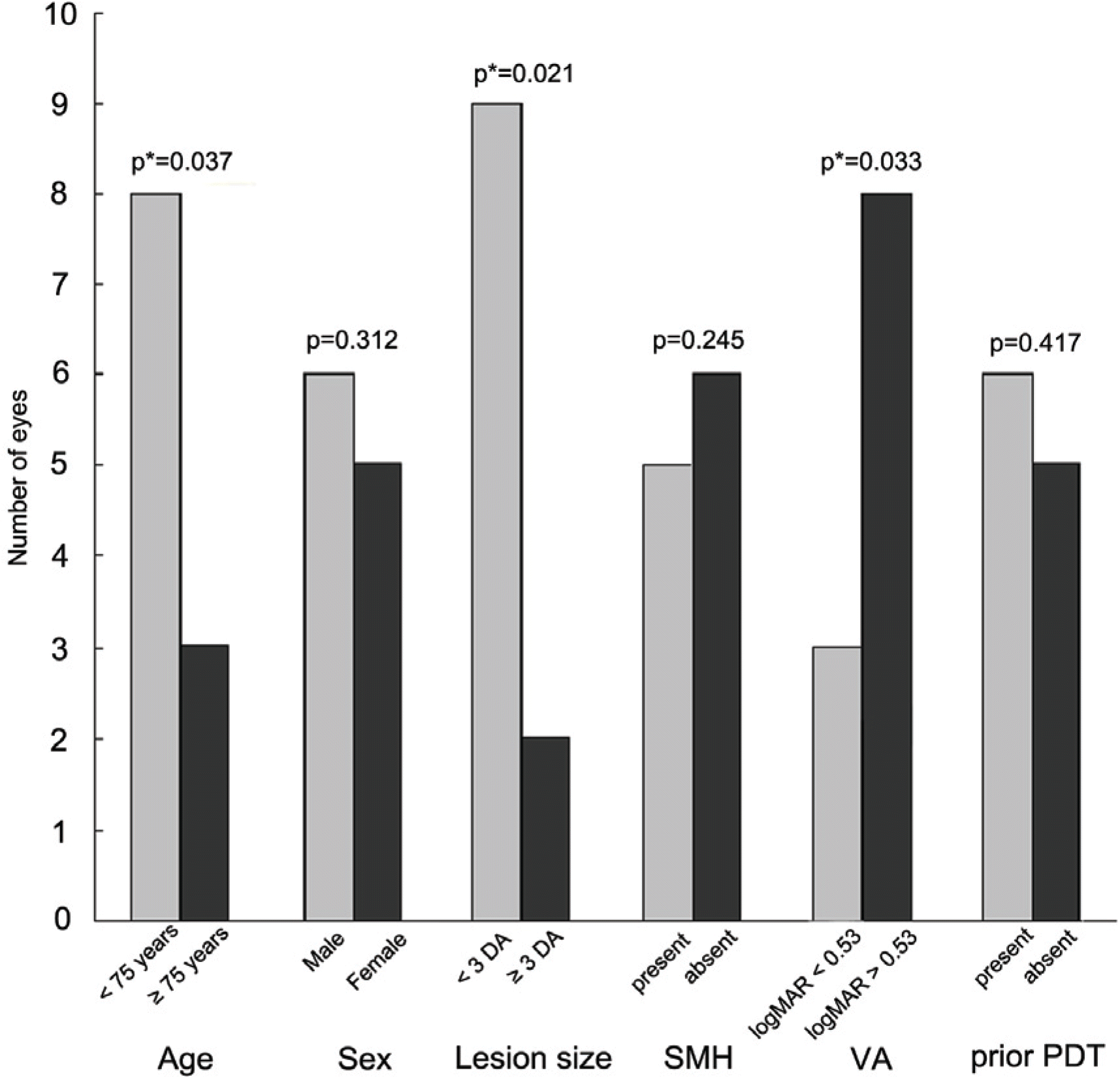 | Figure 4.Comparison of the ratio of subjects gaining 1 line or more at 12-month follow-up depending on * p is age, sex, lesion size, SMH, VA, prior PDT; statistically significant on Chi-square test of variances; DA=disc area; SMH=submacular hemorrhage; logMAR= logarithm of the minimum angle of resolution; VA=visual acuity; PDT=photodynamic therapy. |
Table 1.
Baseline features of patients with subfoveal CNV due to age-related macular degeneration
| Baseline (%) | |
|---|---|
| Eyes | 31 |
| Mean age± SD | 72.3±7.5 |
| < 75 years | 20 (64.5) |
| ≥ 75 years | 11 (35.5) |
| Women | 10 (32.2) |
| CNV* subtype | |
| predominantly classic | 8 (25.8) |
| minimally classic | 1 (3.2) |
| Occult | 22 (71.0) |
| PED† | 10 (32.2) |
| Submacular hemorrhage | 14 (45.2) |
| Size of lesion (DA‡) | |
| < 3 DA | 13 (41.9) |
| ≥ 3 DA | 18 (58.1) |
| Prior PDT§ | 16 (51.6) |
Table 2.
Comparison of the effects depending on various factors
|
VA‡ (logMAR§) |
Foveal thickness (μm) |
Total macular volume (mm3) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| base | 1 mo | 4 mo | 12 mo | base | 1 mo | 4 mo | 12 mo | Base | 1 mo | 4 mo | 12 mo | |||
| < 75 (20 eyes) | 0.65±0.45 | 0.58±0.43 | 0.58±0.45 | 0.58±0.71 | 328±95 | 251±67 | 218±52 | 223±49 | 9.80±3.30 | 7.12±1.40 | 0 6.33±0.76 | 6 6.43±0.59 | ||
| p* | 0.011 | 0.046 | 0.032 | 0.003 | <0.001 | <0.001 | 0.001 | <0.001 | <0.01 | |||||
| Age (years) | ≥ 75 (11 eyes) | 0.91±0.50 | 0.88±0.48 | 0.89±0.47 | 0.88±0.53 | 311±71 | 226±50 | 221±59 | 233±41 | 9.67±3.19 | 6.99±2.23 | 3 6.44±1.43 | 3 6.54±1.11 | |
| p* | 0.180 | 0.285 | 0.341 | 0.003 | 0.016 | 0.011 | 0.003 | 0.006 | 0.014 | |||||
| p† | 0.133 | 0.036 | 0.041 | 0.036 | 0.703 | 0.338 | 0.887 | 0.731 | 0.887 | 0.528 | 0.951 | 0.812 | ||
| Male (20 eyes) | 0.52±0.52 | 0.46±0.50 | 0.45±0.52 | 0.46±0.49 | 290±74 | 220±63 | 216±54 | 228±58 | 8.13±2.85 | 6.77±1.03 | 3 6.36±0.82 | 2 6.59±1.43 | ||
| p* | 0.026 | 0.034 | 0.047 | 0.004 | <0.001 | 0.008 | <0.001 | <0.001 | <0.001 | |||||
| Sex | Female (11 eyes) | 0.70±0.41 | 0.54±0.39 | 0.69±0.38 | 0.68±0.43 | 360±94 | 243±58 | 200±56 | 239±83 | 9.95±3.65 | 6.88±2.42 | 2 6.47±1.36 | 6 6.77±2.01 | |
| p* | 0.048 | 0.197 | 0.127 | 0.003 | 0.013 | 0.049 | 0.003 | 0.006 | 0.019 | |||||
| p† | 0.227 | 0.359 | 0.081 | 0.104 | 0.087 | 0.169 | 0.761 | 0.641 | 0.212 | 0.555 | 0.951 | 0.843 | ||
| < 0.53 (18 eyes) | 0.39±0.15 | 0.35±0.14 | 0.38±0.19 | 0.38±0.10 | 334±91 | 245±67 | 212±50 | 221±61 | 10.26±3.65 | 7.21±1.87 | 7 6.32±1.11 | 1 6.63±1.47 | ||
| Initial VA (logMAR) | p* | 0.034 | 0.422 | 0.571 | 0.003 | 0.001 | 0.007 | 0.001 | 0.001 | 0.001 | ||||
| > 0.53 (13 eyes) | 1.23±0.36 | 0.81±0.38 | 0.82±0.41 | 0.80±0.24 | 306±79 | 239±57 | 230±60 | 247±52 | 9.06±2.45 | 6.89±1.52 | 2 6.43±0.95 | 5 6.77±1.44 | ||
| p* | 0.036 | 0.041 | 0.037 | 0.002 | 0.013 | 0.009 | 0.002 | 0.004 | 0.009 | |||||
| p† | <0.001 | <0.001 | <0.001 | <0.001 | 0.373 | 1.000 | 0.183 | 0.104 | 0.514 | 0.567 | 0.373 | 0.249 | ||
| ≤ 3 (13 eyes) | 0.71±0.46 | 0.64±0.40 | 0.63±0.40 | 0.62±0.31 | 300±72 | 243±70 | 223±68 | 241±41 | 8.82±3.18 | 6.95±2.03 | 3 6.59±1.22 | 2 6.71±1.45 | ||
| p* | 0.016 | 0.044 | 0.032 | 0.023 | 0.016 | 0.021 | 0.002 | <0.001 | 0.001 | |||||
| Lesion size (DA) | 3 – 6 (17 eyes) | 0.52±0.52 | 0.52±0.54 | 0.52±0.56 | 0.53±0.50 | 327±97 | 232±55 | 204±36 | 220±39 | 9.86±3.31 | 6.81±1.45 | 5 6.12±0.82 | 2 6.31±1.49 | |
| p* | 0.109 | 0.680 | 0.209 | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | 0.004 | |||||
| p† | 0.073 | 0.044 | 0.036 | 0.041 | 0.798 | 0.352 | 0.196 | 0.243 | 0.921 | 0.312 | 0.211 | 0.197 | ||
| Present (14 eyes) | 0.67±0.47 | 0.59±0.43 | 0.59±0.41 | 0.60±0.38 | 362±97 | 271±57 | 217±51 | 228±53 | 10.60±4.00 | 7.81±2.03 | 3 6.32±0.79 | 9 6.53±1.01 | ||
| p* | 0.042 | 0.048 | 0.053 | 0.002 | <0.001 | 0.003 | 0.002 | <0.001 | <0.001 | |||||
| SMHΠ | Absent (17 eyes) | 0.81±0.51 | 0.76±0.51 | 0.76±0.50 | 0.77±0.37 | 289±68 | 218±60 | 221±61 | 233±54 | 9.06±2.54 | 6.47±1.29 | 9 6.41±1.27 | 7 6.59±1.04 | |
| p* | 0.039 | 0.049 | 0.051 | 0.004 | 0.013 | 0.019 | <0.001 | 0.002 | 0.005 | |||||
| p† | 0.421 | 0.279 | 0.262 | 0.311 | 0.032 | 0.002 | 0.984 | 0.814 | 0.356 | 0.029 | 0.984 | 0.847 | ||
| Present (16 eyes) | 0.69±0.51 | 0.63±0.51 | 0.62±0.54 | 0.62±31 | 293±56 | 228±49 | 229±52 | 238±47 | 8.56±1.91 | 6.46±0.90 | 0 6.37±0.82 | 2 6.49±0.67 | ||
| p* | 0.041 | 0.039 | 0.043 | <0.001 | 0.004 | 0.007 | <0.001 | 0.002 | 0.010 | |||||
| Prior PDT# | Absent (15 eyes) | 0.80±0.49 | 0.74±0.44 | 0.73±0.44 | 0.73±0.40 | 322±89 | 242±64 | 219±56 | 235±30 | 11.03±4.03 | 7.72±2.23 | 3 6.37±1.31 | 1 6.51±0.97 | |
| p* | 0.041 | 0.046 | 0.048 | <0.001 | <0.001 | <0.001 | 0.003 | 0.002 | 0.005 | |||||
| p† | 0.830 | 0.922 | 0.264 | 0.314 | 0.299 | 0.247 | 0.129 | 0.197 | 0.110 | 0.626 | 0.626 | 0.641 | ||




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download