Abstract
Purpose
To evaluate antioxidative and preventive effects of sea tangle extract on selenite-induced cataract formation.
Methods
Eighty SD rat pups were randomized into 8 groups. Group 1 received no injection of reagent (normal); Group 2 to 8 received injection of selenite (15 μmol/Kg, s.c.) was injected. In group 2 (control) and group 3, normal saline (i.p.) and ascorbic acid (i.p.) was injected on days 3∼31. In groups 4∼8, sea tangle extract (i.p.) was injected at a concentration of 12.5, 25, 50, 100, 200 mg/kg, respectively. Development of cataract was assessed and photographed weekly under slit lamp. Rat lenses were analyzed for antioxidant enzymes, glutathione peroxidase (GPx), superoxide dismutase and malondialdehyde. Furthermore, an amino acid analysis of sea tangle extract was performed.
Results
Significant differences (p <0.05) were seen in cataract development in group 7. Dense nuclear cataracts developed in 8 of 10 of the control group (group 2); Group 4∼8 developed nuclear cataract with proportion of 6/10, 3/10, 2/10, 1/10, and 6/10 rats. In sea tangle injected group, levels of GPx were higher than in the ascorbic acid and control groups. In particular, group 7, injected with 100 mg/kg of sea tangle extract, showed significantly high level of enzyme. Results of the amino acid analysis showed sea tangle includes glutamate-glycine-cysteine, major constituents of glutathione (GSH).
References
1. Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003; 290:2057–60.

2. Resnikoff S, Pascolini D, Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004; 82:844–51.
3. Abraham AG, Condon NG, West Gower E. The new epidemiology of cataract. Ophthalmol Clin North Am. 2006; 19:415–25.
4. Harding J. The epidemiology of cataract. Harding J, editor. Cataract- biochemistry, Epidemiology and Pharmacology. Madras: Chapman & Hall;1991. 2:chap. 13.
7. Reddan JR, Sevilla MD, Giblin FJ, et al. The superoxide dis-mutase mimic TEMPOL protects cultured rabbit lens epithelial cells from hydrogen peroxide insult. Exp Eye Res. 1993; 56:543–54.

8. Kasuya M, Itoi M, Kobayashi S, et al. Changes of glutathione and taurine concentrations in lenses of rat eyes induced by galactose- cataract formation or ageing. Exp Eye Res. 1992; 54:49–53.
9. Sperduto RD, Hu TS, Milton RC, et al. The Linxian cataract studies. Two nutrition intervention trials. Arch Ophthalmol. 1993; 111:1246–53.
10. Mares-Perlman JA, Lyle BJ, Klein R, et al. Vitamin supplement use and incident cataracts in a population-based study. Arch Ophthalmol. 2000; 118:1556–63.

11. Elanchezhian R, Ramesh E, Sakthivel M, et al. Acetyl-L-carnitine prevents selenite-induced cataractogenesis in an experimental animal model. Curr Eye Res. 2007; 32:961–71.

12. Arnal E, Miranda M, Almansa I, et al. Lutein prevents cataract development and progression in diabetic rats. Graefes Arch Clin Exp Ophthalmol. 2009; 247:115–20.

13. Yağ ci R, Aydin B, Erdurmuş M, et al. Use of melatonin to prevent selenite-induced cataract formation in rat eyes. Curr Eye Res. 2006; 31:845–50.
14. Sakthivel M, Elanchezhian R, Ramesh E, et al. Prevention of selenite-induced cataractogenesis in wistar rats by the polyphenol, ellagic acid. Exp Eye Res. 2008; 86:251–9.

15. Park JH, Lee YJ, Kim JJ, et al. The effect of pinitol on cataractogenesis and antioxidative effect in streptozotocin induced diabetic rats. J Korean Ophthalmol Soc. 2005; 46:1886–93.
16. Tang H, Inoue M, Uzawa Y, Kawamura Y. Anti-tumorigenic components of a sea weed, Eeteromorpha clathrata. Biofactors. 2004; 22:107–10.
17. Park PJ, Kim EK, Lee SJ, et al. Protective effects against H2 O2-induced damage by enzymatic hydrolysates of an edible brown seaweed, sea tangle (Laminaria japonica). J Med Food. 2009; 12:159–66.
18. Yan X, Nagata T, Fan X. Antioxidative activities in some common sea weeds. Plant Foods Hum Nutr. 1998; 52:253–62.
19. Rupérez P, Ahrazem O, Leal JA. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J Agric Food Chem. 2002; 50:840–5.

20. Lee KS, Choi YS, Seo JS. Sea tangle supplementation lowers blood glucose and supports antioxidant systems in streptozotocin-induced diabetic rats. J Med Food. 2004; 7:130–5.

21. Ostádalová I, Babický A, Obenberger J. Cataract induced by administration of a single dose of sodium selenite to suckling rats. Experientia. 1978; 34:222–3.

25. Harding JJ. Free and protein-bound glutathione in normal and cataractous human lenses. Biochem J. 1970; 117:957–60.

26. Santini SA, Marra G, Giardina B, et al. Defective plasma antioxidant defenses and enhanced susceptibility to lipid peroxidation in uncomplicated IDDM. Diabetes. 1997; 46:1853–8.

27. Hightower KR, McCready JP. Effect of selenite on epithelium of cultured rabbit lens. Invest Ophtalmol Vis Sci. 1991; 32:406–9.
28. Kumaran S, Savitha S, Anusuya Devi M, Panneerselvam C. Lcarnitine and DL-alpha-lipoic acid reverse the age-related deficit in glutathione redox state in skeletal muscle and heart tissues. Mech Ageing Dev. 2004; 125:507–12.
29. Nishigori H, Lee JW, Iwatsuru M. An animal model for cataract research: cataract formation in developing chick embryo by glucocorticoid. Exp Eye Res. 1983; 36:617–21.

30. Kimura Y, Watanabe K, Okuda H. Effects of soluble sodium alginate on cholesterol excretion and glucose tolerance in rats. J Ethnopharmacol. 1996; 54:47–54.

31. Kim JP. Development of protein utilization from insoluble algae-I. Korean J Food Sci Technol. 1974; 6:17–23.
32. Okumura A, Oishi K, Murata K. Quality of KOMBU, one of the edible seaweeds, belonging to the Laminariaceae-Ⅶ. water- extracting conditions of total and amino nitrogens. Bull Japan Fish Sci Soc. 1963; 29:1089–91.
33. Bhat KS, John A, Reddy PR, et al. Effect of pigmentation on glutathione redox cycle antioxidant defense in whole as well as different regions of human cataractous lens. Exp Eye Res. 1991; 52:715–21.

34. Lim J, Li L, Jacobs MD, et al. Mapping of glutathione and its precursor amino acids reveals a role for GLYT2 in glycine uptake in the lens core. Invest Ophthalmol Vis Sci. 2007; 48:5142–51.

35. Hockwin O, Hata N. Changes in the content in reduced and oxydized glutathione in incubated bovine lenses. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1978; 206:151–5.
Figure 1.
Development selenite-induced cataract on the 4th day after sodium selenite injection. (A) External photograph of cataract development (B) Slit lamp photograph of cataract development. The central dense nuclerosclerosis cataract was found after sodium selenite injection.
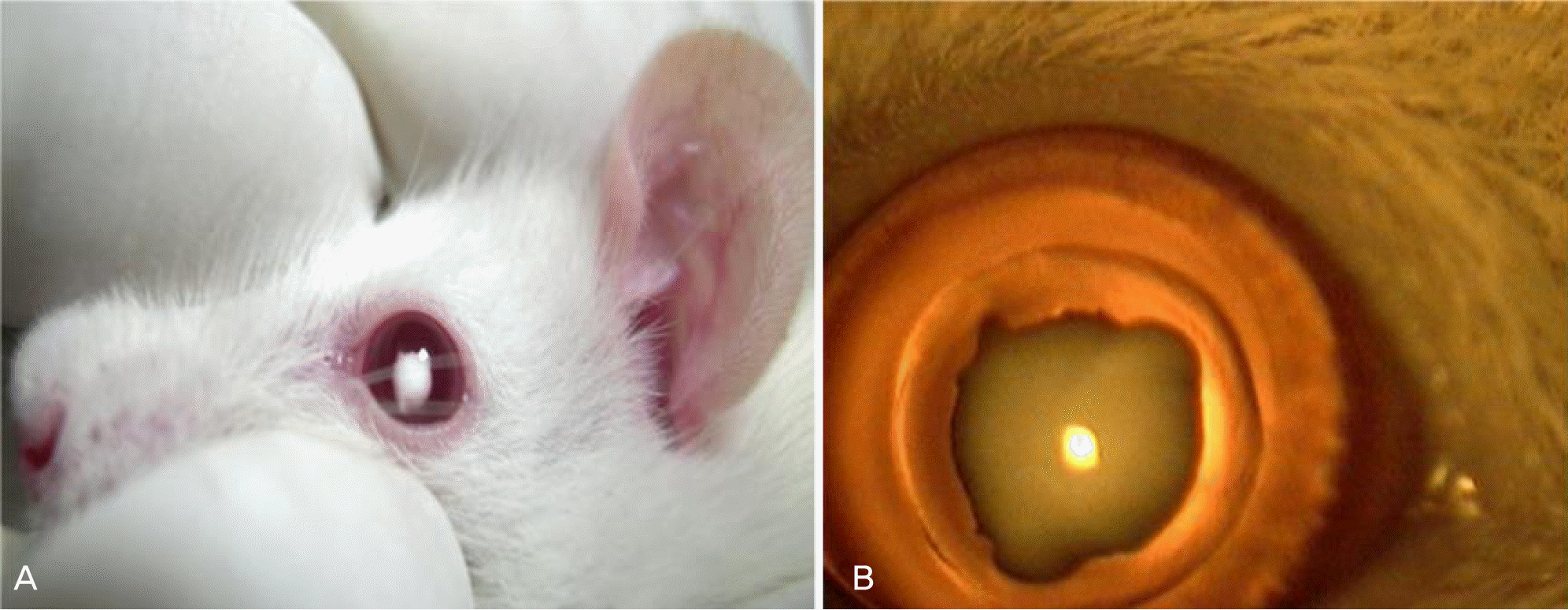
Figure 2.
Slit lamp photograph of group 2 (control group) at the final follow-up (3 weeks after sodium selenite injection). Eight of 10 rats developed central nuclear cataract.
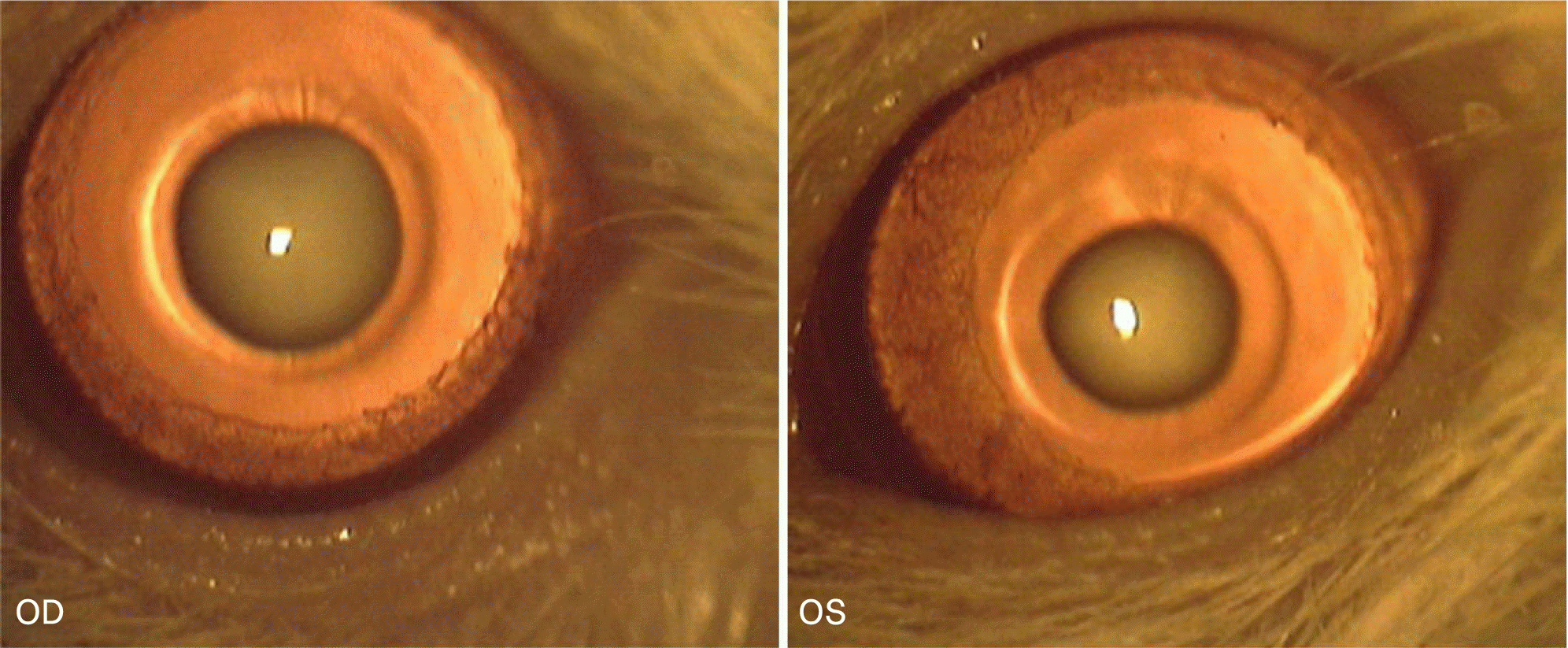
Figure 3.
Slit lamp photograph of group 7 (sea tangle extract 100 mg/kg injected) at the final follow-up (3 weeks after sodium selenite injection,). One of 10 rats developed central nuclear cataract as seen in the two top photographs and remaining 9 rats kept clear lenses as seen in the two bottom photographs.

Table 1.
The incidence of cataract development in each group. Group 7 (sea tangle extract 100 mg/kg) shows the lowest incidence of cataract. As the concentration of sea tangle extract is higher, the ratio of cataract development is getting lower. However, the incidence of cataract is getting high when the concentration is too low or too high (eg. Group 4 and 8)
| Experimental groups | No. of rats | Number of rats in which lenticular opacification occurred | Incidence (%) |
|---|---|---|---|
| Group 1 | 10 | 0 | 0 |
| Group 2 | 10 | 8 | 80 |
| Group 3 | 10 | 4 | 40 |
| Group 4 | 10 | 6 | 60 |
| Group 5 | 10 | 3 | 30 |
| Group 6 | 10 | 2 | 20 |
| Group 7* | 10 | 1* | 10* |
| Group 8 | 10 | 6 | 60 |
Group 1=normal rat pups, Group 2=Selenite+intraperitoneal (i.p) Saline injection, Control group, Group 3=Selenite+i.p Ascorbic acid 1,000 mg/kg injection, Group 4=Selenite+i.p Sea tangle extract 12.5 mg/kg injection, Group 5=Selenite+i.p Sea tangle extract 25 mg/kg injection, Group 6=Selenite+i.p Sea tangle extract 50 mg/kg injection, Group 7=Selenite+i.p Sea tangle extract 100 mg/kg injection, Group 8=Selenite+i.p Sea tangle extract 200 mg/kg injection.
Table 2.
Quantitative analysis of antioxidant system enzymes and malondialdehyde in lenses of rats
| Experimental groups |
Antioxidant enzymes |
MDA (nmol/g) | |
|---|---|---|---|
| GPx (nmol/min/ml) | SOD (U/ml) | ||
| Group 1 | 293.41±12.3 | 62.20±7.3 | 60.5±3.7 |
| Group 2 | 207.72±9.6 | 38.15±6.8 | 88.6±5.3 |
| Group 3 | 210.55±11.2 | 44.75±8.3 | 76.2±6.2 |
| Group 4 | 212.38±7.9 | 44.84±5.7 | 72.5±7.4 |
| Group 5 | 209.91±8.5 | 46.71±11.6 | 74.1±6.8 |
| Group 6 | 230.64±13.2* | 50.10±9.3* | 65.4±7.6* |
| Group 7 | 289.27±9.2* | 52.95±8.9* | 63.2±8.3* |
| Group 8 | 211.72±10.4 | 50.10±7.9* | 69.7±4.8 |
Table 3.
Amino acid analysis of sea tangle extract. Results of amino acid analysis show sea tangle includes glutamate, cysteine and glycine which are components of glutathione




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download