Abstract
Purpose
To evaluate the efficacy and intraocular lens (IOL) stability of vitrectomy combined with the surgical removal of anterior capsular opacity (ACO) and posterior capsular opacity (PCO).
Methods
Forty-four pseudophakic eyes of 43 patients with retinal disorders underwent vitrectomy with surgical removal after cataract diagnosis. Nineteen eyes of 19 patients (group 1) had ACO and PCO removed while 25 eyes of 24 patients (group 2) had only PCO removed. Total removal of the entire lens capsule, except for the area around the haptics, was performed when capsular opacity with fibrosis was observed around the capsulorrhexis margin. LogMAR best corrected visual acuity (BCVA), intraoperative complications and postoperative complications were compared.
Results
The retina was flat and postoperative BCVA improved in both groups. Intraoperative complications of IOL dislocation occurred in 3 eyes (15.8%) in group 1 and in 1 eye (4.0%) in group 2 (p=0.178). Among the 4 IOLs, 3 were open-loop haptic IOLs and 1 was a closed-loop haptic IOL. Late postoperative complications of IOL capture occurred in 1 eye (5.3%) in group 1.
Conclusions
Removal of ACO and PCO for better visualization of the peripheral retina resulted in an improved visual recovery while intraoperative complications and postoperative complications were comparable to PCO removal alone. Removal limited to the optic zone would be more stable when considering any adhesion between the lens capsule and the IOL.
Go to : 
References
1. Machemer R. The development of pars plana vitrectomy: a personal account. Graefes Arch Clin Exp Ophthalmol. 1995; 233:453–68.
2. Campo RV, Sipperley JO. Anterior hyaloidal fibrovascular proliferation after diabetic vitrectomy. Am J Ophthalmol. 1988; 105:432–4.

3. Gündüz K, Bakri SJ. Management of proliferative diabetic retinopathy. Compr Ophthalmol Update. 2007; 8:245–56.
4. Lewis H, Abrams GW, Williams GA. Anterior hyaloidal fibrovascular proliferation after diabetic vitrectomy. Am J Ophthalmol. 1987; 104:607–13.

5. Pollack A, Landa G, Kleinman G, et al. Results of combined surgery by phacoemulsification and vitrectomy. Isr Med Assoc J. 2004; 6:143–6.
6. Nawrocki J, Cisiecki S. Combined surgery, phacoemulsification, implantation of intraocular lens and pars plana vitrectomy. Klin Oczna. 2004; 106:596–604.
7. Wensheng L, Wu R, Wang X, et al. Clinical complications of combined phacoemulsification and vitrectomy for eyes with coexisting cataract and vitreoretinal diseases. Eur J Ophthalmol. 2009; 19:37–45.

8. Ogino N, Kumagai K. Advantage of combined procedure in vitreous surgery. Semin Ophthalmol. 2001; 16:137–8.

9. Apple DJ, Solomon KD, Tetz MR, et al. Posterior capsule opacification. Surv Ophthalmol. 1992; 37:73–116.

10. Kappelhof JP, Vrensen GF. The pathology of after-cataract. A minireview. Acta Ophthalmol Suppl. 1992; 205:13–24.
11. McDonnell PJ, Zarbin MA, Green WR. Posterior capsule opacification in pseudophakic eyes. Ophthalmology. 1983; 90:1548–53.

12. Vasavada AR, Raj SM. Anterior capsule relationship of the AcrySof intraocular lens optic and posterior capsule opacification: a prospective randomized clinical trial. Ophthalmology. 2004; 111:886–94.
14. Kim SH, Chung JW, Chung H, Yu HG. Phacoemulsification and foldable intraocular lens implantation combined with vitrectomy and silicone oil tamponade for severe proliferative diabetic retinopathy. J Cataract Refract Surg. 2004; 30:1721–6.

15. Weinreb RN, Wasserstrom JP, Parker W. Neovascular glaucoma following neodymium-YAG laser posterior capsulotomy. Arch Ophthalmol. 1986; 104:730–1.

16. Poliner LS, Christianson DJ, Escoffery RF, et al. Neovascular glaucoma after intracapsular and extracapsular cataract extraction in diabetic patients. Am J Ophthalmol. 1985; 100:637–43.

17. Park SE, Lee SJ. Mechanized posterior capsulectomy during combined vitrectomy and cataract surgery. J Korean Ophthalmol Soc. 2007; 48:1335–40.

Go to : 
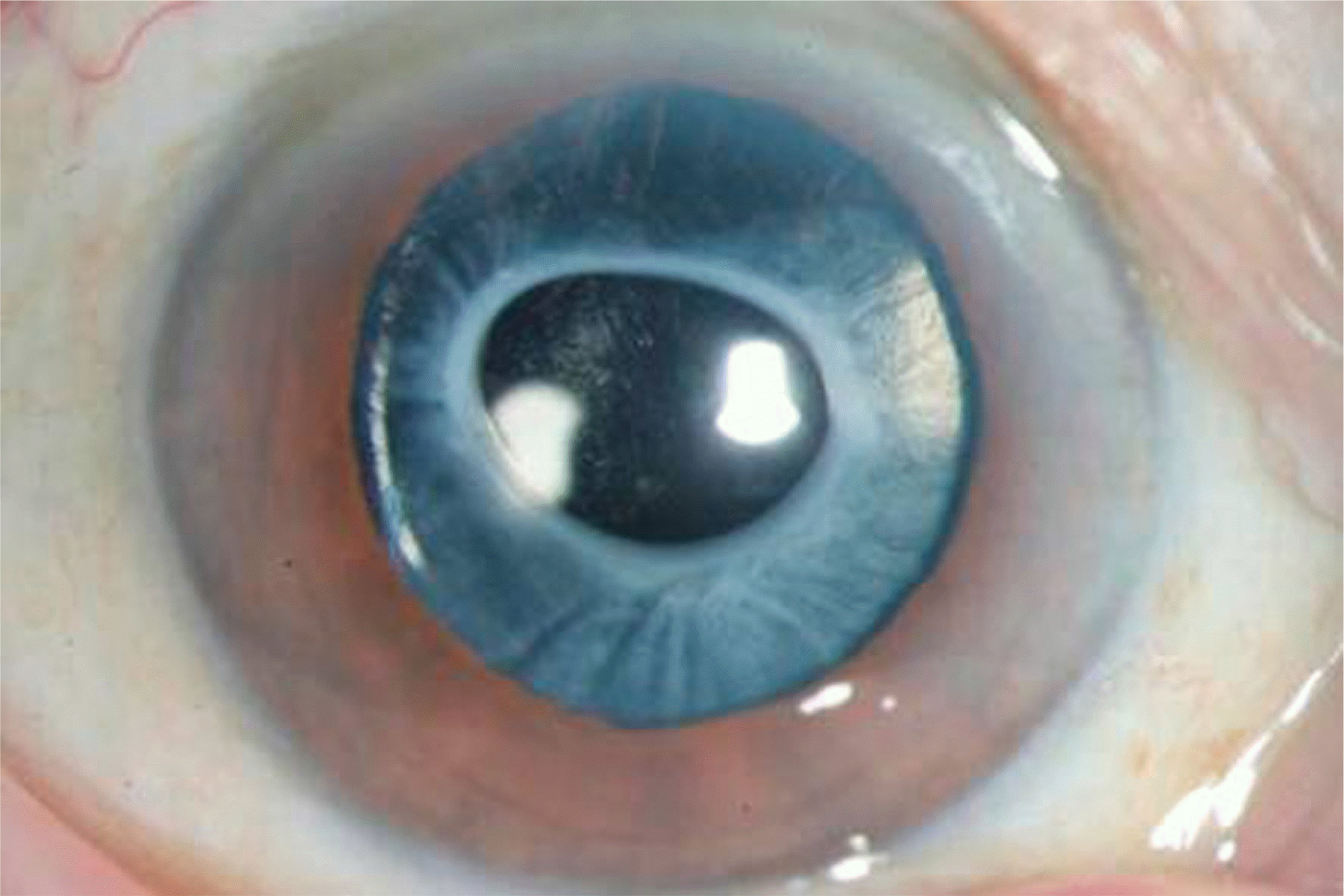 | Figure 1.Anterior segment photograph of a patient with anterior and posterior capsular opacity showing a dense fibrotic change around the contractured capsulorrhexis margin. |
 | Figure 2.Surgical removal of anterior capsular opacity (ACO). (A) A broad ACO was seen preoperatively. (B) One half of the anterior capsule was cut using a bent 26G needle after confirming the degree of adhesion between the lens capsule and the IOL. (C) Another half was cut from the opposite side. (D) Then, the anterior capsule was removed through the corneal entry site. After ACO removal, the posterior capsular opacity was removed with vitreous cutter. |
 | Figure 3.Intraocular lens (IOL) stability depending on the IOL type. Anterior and posterior capsular opacity in a 3 piece open-loop haptic IOL (A) and a single piece closed-loop haptic IOL (B). The postoperative photograph of closed-loop haptic IOL seems to be more stable with its wide haptic portion (D) than the open-loop haptic IOL (C). The arrows indicate the remnant lens capsule at the hapatic portion. |
Table 1.
Demographics of the patients
| Group 1 (n=19) | Group 2 (n=25) | p value | |
|---|---|---|---|
| Age (years) | 59.21±13.15 | 64.04±8.66 | 0.103 |
| F/U (months) | 12.05±14.75 | 13.56±18.14 | 0.424 |
| Preop logMAR VA | 1.53±1.08 | 1.38±1.06 | 0.796 |
| Postop logMAR VA | 0.74±0.39 | 0.95±0.81 | 0.043∗ |
| Proliferative diabetic retinopathy | 12 (63.2%) | 14 (56.0%) | 0.632 |
| Macular hole | 2 (10.5%) | 6 (24.0%) | 0.251 |
| Epiretinal membrane | 2 (10.5%) | 3 (12.0%) | 0.879 |
| Rhegmatogenous retinal detachment | 2 (10.5%) | 2 (8.0%) | 0.773 |
| Vitreous opacity | 1 (5.3%) | 0 (0%) | 0.246 |
| Gas tamponade | 10 (52.6%) | 16 (64.0%) | 0.447 |
| Scleral buckling/encircling | 4 (21.1%) | 5 (20.0%) | 0.932 |
Table 2.
Types of implanted IOLs
| Group 1 (n=19) | Group 2 (n=25) | ||
|---|---|---|---|
| 1 piece IOL | 570C (Rayner) | 8 (1∗) | 9 |
| 604A (Rayner) | 2 | 1 | |
| 3 piece IOL | AR40e (Advanced Medical Optics) | 7 (1∗) | 12 (1∗) |
| Silens6 (Bausch & Lomb) | 1 (1∗) | 2 | |
| SI40NB (Advanced Medical Optics) | 1 | 1 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download