Abstract
Purpose
To investigate the short-term effect of intravitreal bevacizumab injection for choroidal neovascularization associated with degenerative myopia.
Methods
In 15 eyes of 15 patients, one or two consecutive intravitreal bevacizumab injections were given. The best-corrected visual acuity (BCVA) and fundus examination were evaluated at baseline and monthly thereafter. Fluorescence angiography (FA) was performed at baseline, 1 month and 3 months after treatment. When the angiographic leakage persisted 1 month after the first injection, a second injection was administered.
Results
The mean followup period was 9.7 months. The mean logarithm of the minimum angle of resolution (LogMAR) BCVA was 0.81±0.44 at baseline, 0.64±0.41 at 3 months (p=0.005), and 0.60±0.41 (p=0.001) at the final examination. Five eyes received a single injection, while the other ten eyes had two consecutive injections. Three months after the first injection, 14 eyes (93.3%) had no angiographic leakage, and 1 eye (6.7%) showed a decrease in leakage. The mean lines of visual improvement at 3 months and at the final examination were 1.7 and 2.1 lines, respectively. No case of vision loss was observed throughout the followup period.
References
1. Grossniklaus HE, Green WR. Pathologic finding in pathologic myopia. Retina. 1992; 12:127–33.
2. Hotchkiss ML, Fine SL. Pathologic myopia and choroidal neovascularization. Am J Ophthalmol. 1981; 91:177–83.

3. Ohno-Matsui K, Yoshida T, Futagami S, et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularization in pathological myopia. Br J Ophthalmol. 2003; 87:570–3.
4. Hampton GR, Kohen D, Bird AC. Visual prognosis of disciform degeneration in myopia. Ophthalmology. 1983; 90:923–6.

5. Yoshida T, Ohno-Matsui K, Yasuzumi K, et al. Myopic choroidal neovascularization. A 10-year followup. Ophthalmology. 2003; 110:1297–305.
6. Secretan M, Kuhn D, Soubrane G, Coscas G. Long-term visual outcome of choroidal neovascularization in pathologic myopia; natural history and laser treatment. Eur J Ophthalmol. 1997; 7:307–16.
7. Johnson DA, Yannuzzi LA, Shakin JL, Lightman DA. Lacquer cracks following laser treatment of choroidal neovascularization in pathologic myopia. Retina. 1998; 18:118–24.

8. Bottoni F, Perego E, Airaghi P, et al. Surgical removal of subfoveal choraoidal neovascular membranes in high myopia. Graefes Arch Clin Exp Ophthalmol. 1999; 237:573–82.
9. Uemura A, Thomas MA. Subretinal surgery for choroidal neovascularization in patients with high myopia. Arch Ophthalmol. 2000; 118:344–50.

10. Glacet-Bernard A, Simon P, Hamelin N, et al. Translocation of the macula for management of subfoveal choroidal neovascularization: comparision of results in age-related macular degeneration and degenerative myopia. Am J Ophthalmol. 2001; 131:78–89.
11. Ichibe M, Imai K, Ohta M, et al. Foveal translocation with scleral imbrication in patients with myopic neovascular maculopathy. Am J Ophthalmol. 2001; 132:164–71.

12. Verteporfin in Photodynamic Therapy (VIP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin: 1-year results of a randomized clinical trial-VIP report no. 1. Ophthalmology. 2001; 108:841–52.
13. Verteporfin in Photodynamic Therapy (VIP) Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomizedclinical trial-VIP report no. 3. Ophtalmology. 2003; 110:667–73.
14. Ryu IH, Kim BG, Lee SC. Photodynamic Therapy of Subfoveal Choroidal Neovascularization in Pathologic Myopia. J Korean Ophthalmol Soc. 2003; 44:1991–5.
15. Chung EJ, Oh HS, Koh HJ, et al. Photodynamic therapy in practice: A review of experiences with myopic CNV in Korean patients. J Korean Ophthalmol Soc. 2005; 46:664–70.
16. Schnurrbusch UE, Jochmann C, Wiedemann P, Wolf S. Quan-titative assessment of the long-term effect of photodynamic therapy in patients with pathologic myopia. Graefes Arch Clin Exp Opththalmol. 2005; 243:829–33.

17. Lam DS, Liu DT, Fan DS, et al. Photodynamic therapy with verteporfin for juxtafoveal choroidal neovascularization secondary to pathologic myopia-1-year results of a prospective series. Eye. 2005; 19:834–40.

18. Pece A, Isola V, Vadalà M, Matranga D. Photodynamic therapy with verteporfin for subfoveal choroidal neovascularization secondary to pathologic myopia: long-term study. Retina. 2006; 26:746–51.
19. Pece A, Vadalà M, Isola V, Matranga D. Photodynamic therapy with verteporfin for juxtafoveal choroidal neovascularization in pathologic myopia: a long-term followup study. Am J Ophthalmol. 2007; 143:449–54.

20. Degenring RF, Jonas JB. Photodynamic therapy in combination with intravitreal triamcinolone for myopic choroidal neovascularization. Acta Ophthalmol Scand. 2005; 83:621.

21. Chan WM, Lai TY, Wong AL, et al. Combined photodynamic therapy and intravitreal triamcinolone injection for the treatment of choroidal neovascularization secondary to pathological myopia: a pilot study. Br J Ophthalmol. 2007; 91:174–9.
22. Sakaguchi H, Ikuno Y, Gomi F, et al. Intravitreal injection of bevacizumab for choroidal neovascularization associated with pathological myopia. Br J Ophthalmol. 2007; 91:161–5.
23. Yamamoto I, Rogers AH, Reichel E, et al. Intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularization secondary to pathological myopia. Br J Ophthalmol. 2007; 91:157–60.
24. Chan WM, Lai TY, Liu DT, Lam DS. Intravitreal bevacizumab (Avastin) for myopic choroidal neovascularization: six-month results of a prospective pilot study. Ophthalmology. 2007; 114:2190–6.
25. Ruiz-Moreno JM, Gomez-Ulla F, Montero JA, et al. Intravitreous bevacizumab to treat subfoveal choroidal neovascularization in highly myopic eyes: short-term results. Eye. 2009; 23:334–8.

26. Mandal S, Venkatesh P, Sampangi R, Garg S. Intravitreal bevacizumab (Avastin) as primary treatment for myopic choroidal neovascularization. Eur J Ophthalmol. 2007; 17:620–6.

27. Hernandez-Rojas ML, Quiroz-Mercado H, Dalma-Weiszhausz J, et al. Short-term effects of intravitreal bevacizumab for subfoveal choroidal neovascularization in pathologic myopia. Retina. 2007; 27:707–12.
28. Steidl SM, Pruett RC. Macular complication associated with posterior staphyloma. Am J Ophthalmol. 1997; 123:181–7.
29. Brancato R, Trabucchi G, Introini U, et al. Indocyanine green angiography (ICGA) in pathologic myopia. Eur J Ophthalmol. 1996; 16:39–43.
30. Avila MP, Weitter JJ, Jalkh AE, et al. Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology. 1984; 91:1573–81.

31. Song KY, Kim HK, Kim HC. Clinical features of Choroidal Neovascularization in Patient with High Myopia. J Korean Ophthalmol Soc. 2001; 42:983–90.
32. Shahar J, Avery RL, Heilweil G, et al. Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin). Retina. 2006; 26:262–9.

Figure 1.
A 62-year-old woman visited our clinic with metamorphopsia on the left eye (Case 15). At baseline, fundus photograph shows small amount of subretinal hemorrhage at the temporally juxtafoveal area and the nasally juxtafovel area (A). Fluorescein angiography (FA) shows hyperfluorescence in the early phase and increased leakage at the late phase from the nasally and temporally myopic CNV (B, C). At 3 months after single injection of intravitreal bevacizumab, fundus photograph shows resolution of the subretinal hemorrhage with RPE change in the myopic CNV (D). FA shows no evidence of leakage from the previous myopic CNV (E, F).
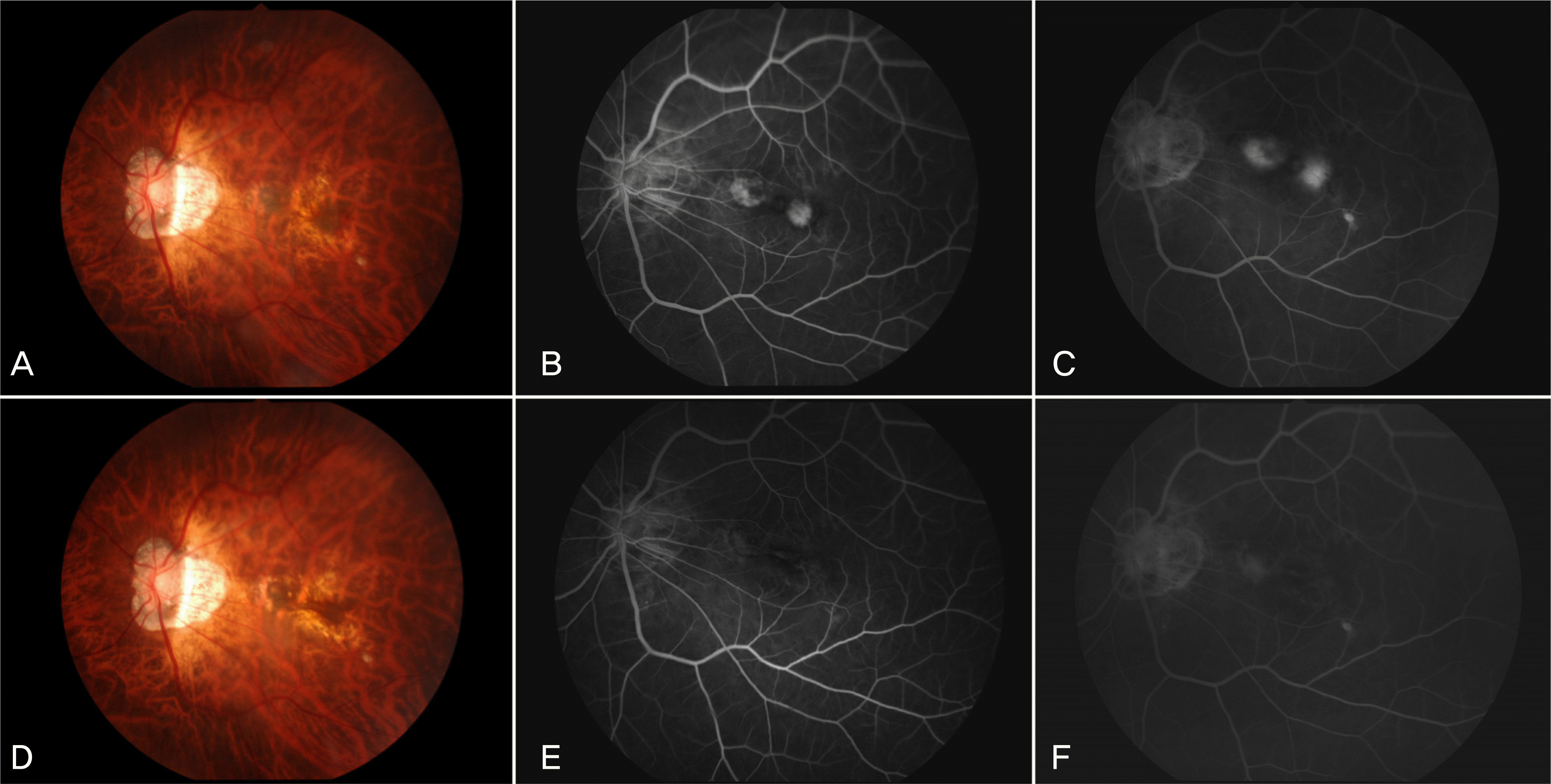
Figure 2.
A 37-year-old woman visited our clinic with decreased visual acuity in the right eye (Case 2). At baseline, fundus photograph shows subretinal hemorrhage at the juxtafoveal area (A). Fluorescein angiography (FA) shows mild leakage atthe early phase and increased leakage at the late phase and (B, C). At 3 months after two consecutive injections of intravitreal bevacizumab, fundus photograph shows resolution of the retinal hemorrhage with fibrosis of the myopic CNV (D). FA shows no leakage but staining at the late phase (E, F).
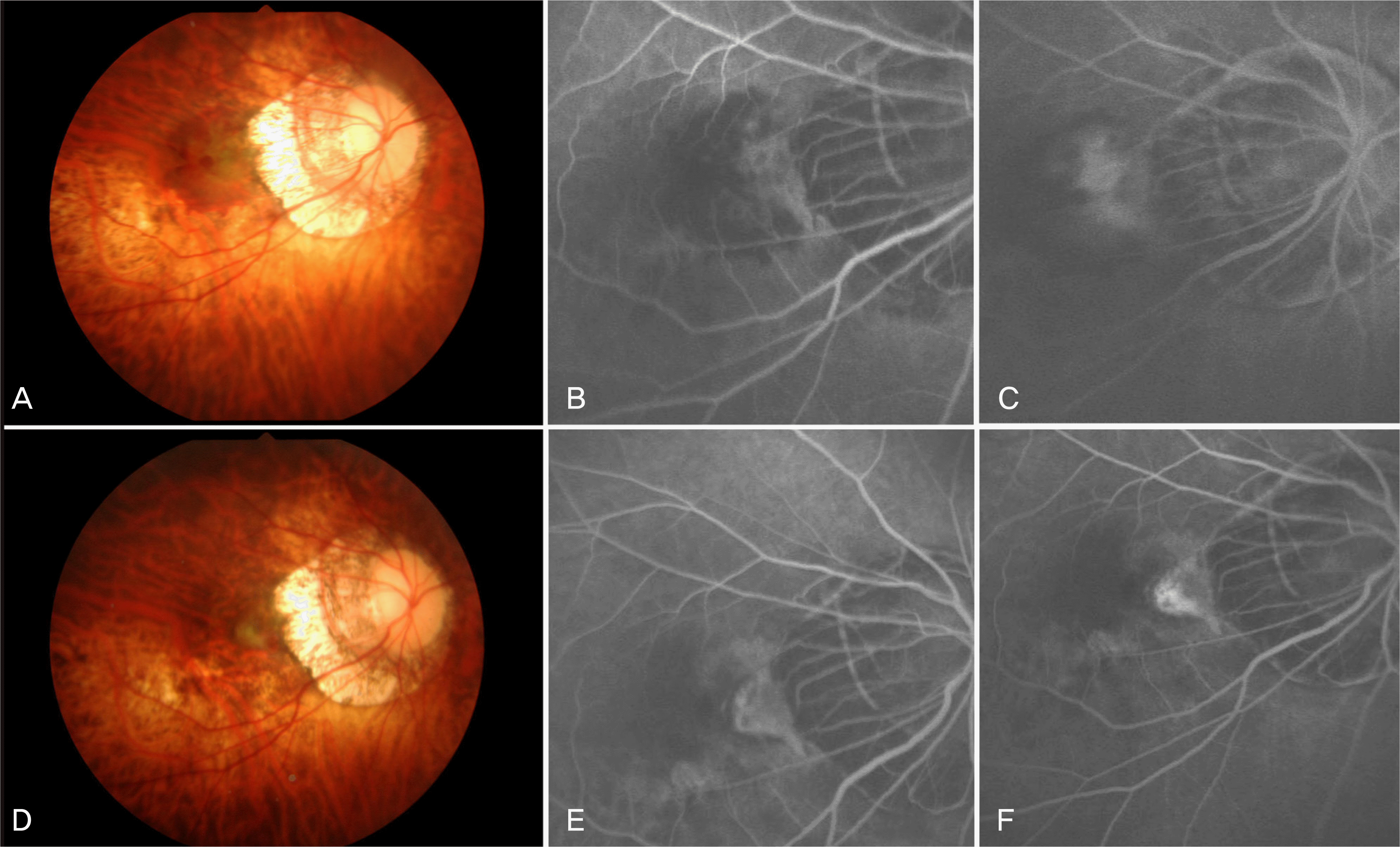
Figure 3.
Showing changes in the mean logarithm of the minimal angle of resolution (logMAR) best corrected visual acuity (BCVA) compared baseline with 3 months and final followup (∗ p=p value<0.05 was considered to be statistically significant).
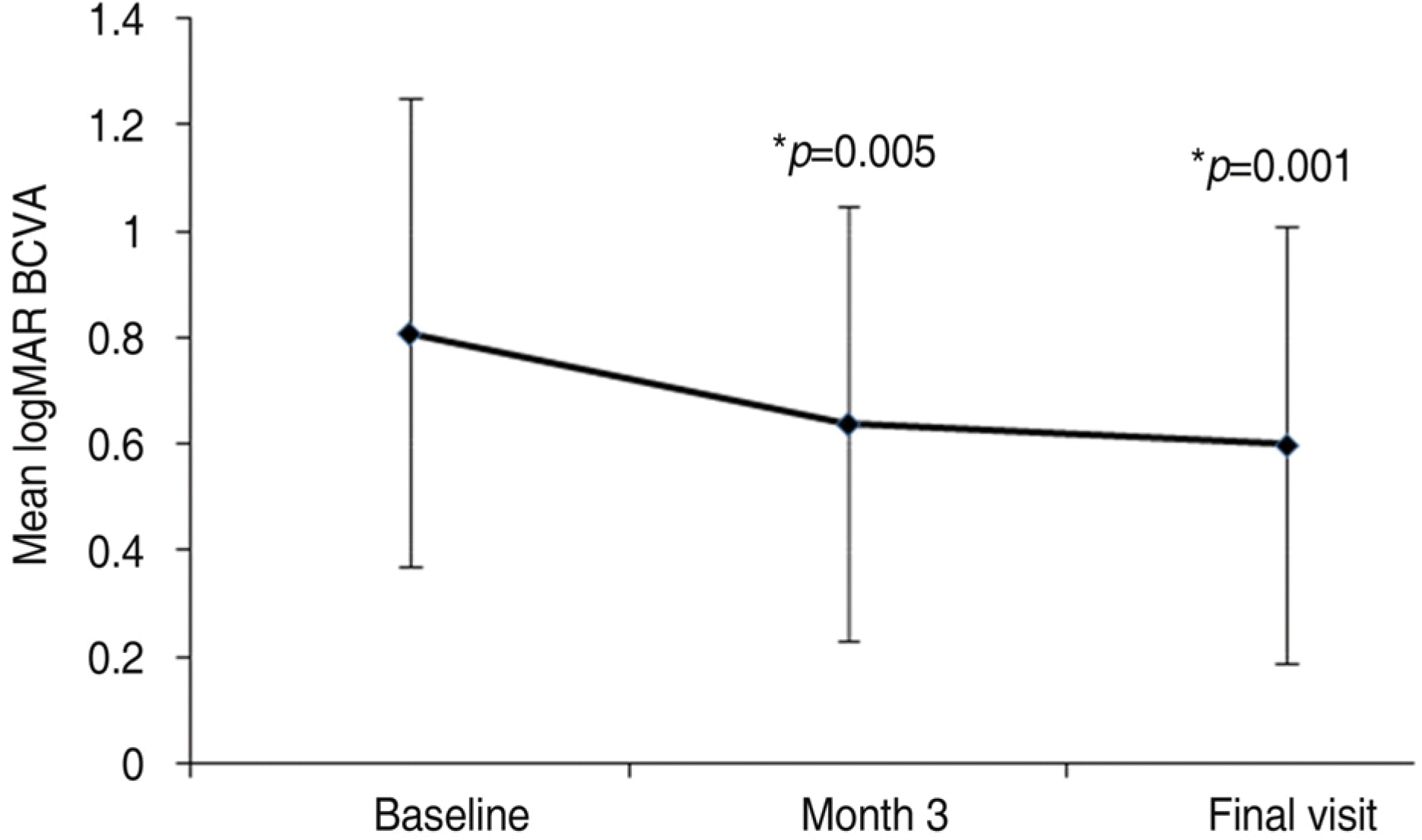
Figure 4.
Change in visual acuity at final examination after initial bivacizumab injection. All thirteen dots below the line indicate eyes with improved vision. Two eyes had stable visual acuity at baseline and 3 months (logMAR=logarithm of the minimal angle of resolution).
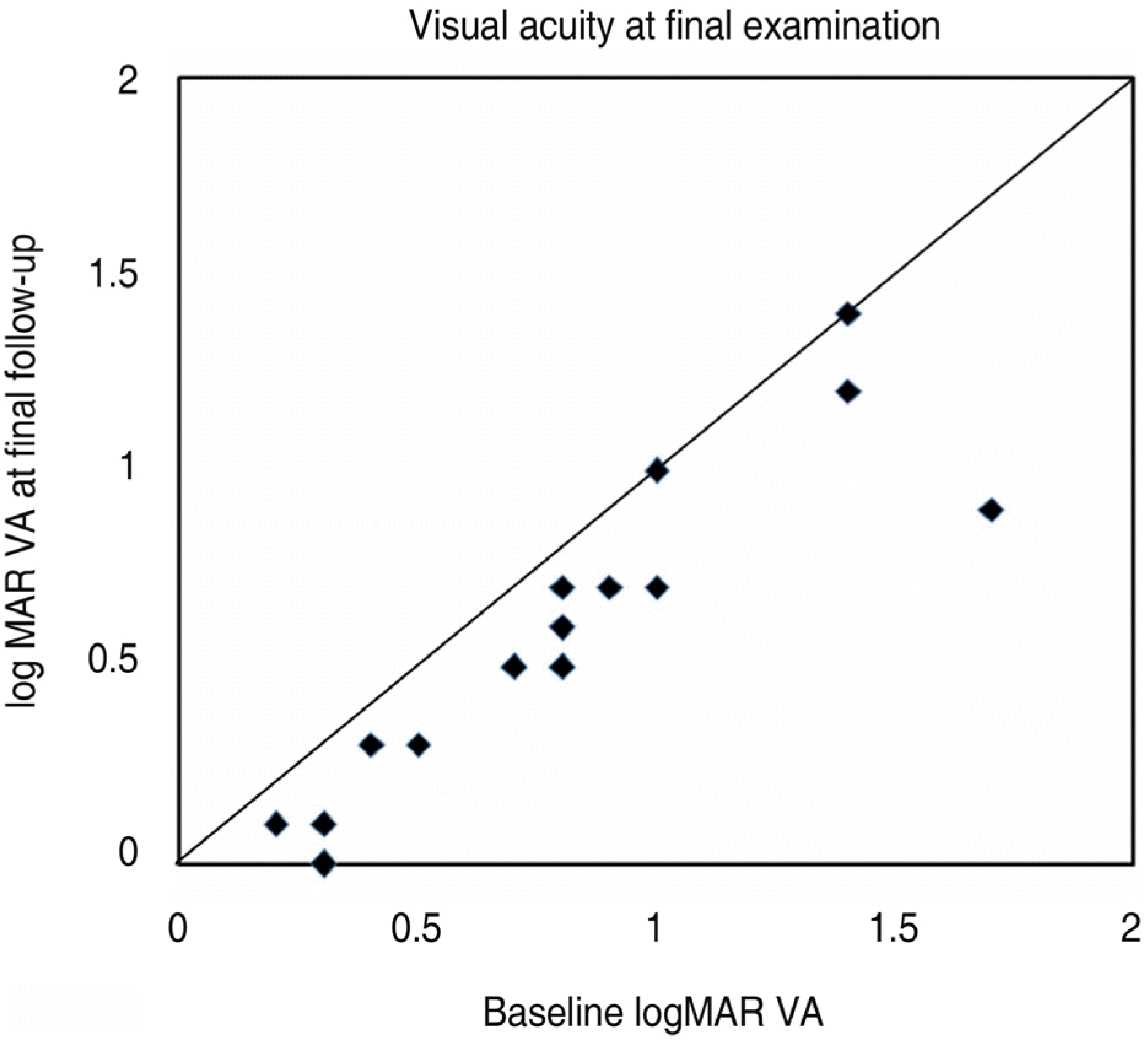
Table 1.
Demographic data of patients with myopic choroidal neovascularization
| Pt. | Age/Sex | F/U (m∗) | Ocular past history | Spherical equivalent (AL†:mm) |
logMAR BCVA‡ |
No. of injection | FA§ leakage (Month 3) | ||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Month 3 | Final | |||||||
| 1 | 51/F | 11 | -17.00 (31.28) | 0.9 | 0.9 | 0.7 | 2 | No | |
| 2 | 37/F | 10 | -26.75 (32.75) | 1 | 1 | 1 | 2 | No | |
| 3 | 42/M | 7 | PRK | -2.25 (28.75) | 0.2 | 0.2 | 0.1 | 2 | Decreased |
| 4 | 80/F | 10 | PDT×1 | -1.38 (29.62) | 1.4 | 1.4 | 1.4 | 2 | No |
| 5 | 77/F | 8 | Lens extraction | 1.38 (28.74) | 1 | 0.7 | 0.7 | 1 | No |
| 6 | 71/F | 9 | Cataract surgery | -1.00 (30.12) | 0.8 | 0.7 | 0.7 | 1 | No |
| 7 | 73/F | 12 | -8.13 (29.78) | 0.8 | 0.5 | 0.5 | 2 | No | |
| 8 | 46/F | 7 | LASIK PDT+IVTA×1 | -2.63 (28.84) | 0.4 | 0.3 | 0.3 | 2 | No |
| 9 | 40/F | 9 | -17.75 (31.75) | 0.5 | 0.3 | 0.3 | 2 | No | |
| 10 | 24/M | 8 | -9.75 (29.67) | 0.3 | 0 | 0 | 2 | No | |
| 11 | 42/M | 10 | PDT× IVTA×1 | -2.63 (27.45) | 0.8 | 0.8 | 0.6 | 1 | No |
| 12 | 62/F | 12 | PDT×1 | -9.75 (28.77) | 1.7 | 0.9 | 0.9 | 2 | No |
| 13 | 65/F | 15 | Cataract surgery | -1.50 (28.43) | 1.4 | 1.2 | 1.2 | 2 | No |
| 14 | 40/F | 10 | Cataract surgery | -24.5 (32.74) | 0.7 | 0.6 | 0.5 | 1 | No |
| 15 | 62/F | 8 | Cataract surgery | 0.50 (27.46) | 0.3 | 0.1 | 0.1 | 1 | No |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download