Abstract
Purpose:
To evaluate the efficacy of preoperative intravitreal bevacizumab (AvastinⓇ; Genetech, San Francisco, CA, USA) injections of pars plana vitrectomy (PPV) for proliferative diabetic retinopathy (PDR).
Methods:
Thirty patients (30 eyes) who underwent PPV for treatment of PDR and received a preoperative intravitreal bevacizumab injection of 1.25 mg were retrospectively analyzed. The study group (group 1, 30 patients, 30 eyes) was compared with a control group (group 2, 29 patients, 30 eyes and matched with the study group for preoperative parameters) who underwent PPV without preoperative intravitreal bevacizumab injection.
Results:
In both groups, visual acuity improved but there was no statistical significance. Intraoperative vitreous hemorrhage occurred in 14 eyes (46.7%) from group 1 and 11 eyes (36.7%) from group 2. There was no statistical significance of intraoperative bleeding occurrence (p=0.3). Postoperative vitreous hemorrhage occurred in 4 eyes from group 1 and 14 eyes from group 2. The group 1 had a lower incidence of postoperative hemorrhage than group 2 (p=0.005).
Go to : 
References
1. Chen E, Park CH. Use Of Intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina. 2006; 26:699–700.

2. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005; 36:331–5.

3. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006; 113:363–72.

4. Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina. 2006; 26:352–4.

5. Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006; 26:275–8.

6. Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmology. 1998; 105:998–1003.

7. Rice TA, Michels RG. Long-term anatomic and functional results of vitrectomy for diabetic retinopathy. Am J Ophthalmol. 1980; 90:297–303.

8. Spaide RF, Fisher YL. Intravitreal bevacizumab (avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006; 26:275–8.

9. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004; 350:2335–42.

10. Schachat AP, Oyakawa RT, Michels RG, et al. Complications of vitreous surgery for diabetic retinopathy. II. Postoperative comp-lications. Ophthalmology. 1983; 90:522–30.
11. Novak MA, Rice TA, Michels RG, Auer C. Vitreous hemorrhage after vitrectomy for diabetic retinopathy. Ophthalmology. 1984; 91:1485–9.

12. West JF, Gregor ZJ. Fibrovascular ingrowth and recurrent haemorrhage following diabetic vitrectomy. Br J Ophthalmol. 2000; 84:822–5.

13. Yang CM, Yeh PT, Yang CH. Intravitreal long-acting gas in the prevention of early postoperative vitreous hemorrhage in diabetic vitrectomy. Ophthalmology. 2007; 114:710–5.

14. Bhende M, Agraharam SG, Gopal L, et al. Ultrasound biomicro-scopy of sclerotomy sites after pars plana vitrectomy for diabetic vitreous hemorrhage. Ophthalmology. 2000; 107:1729–36.
15. Hershberger VS, Augsburger JJ, Hutchins RK, et al. Fibro-vascular ingrowth at sclerotomy sites in vitrectomized diabetic eyes with recurrent vitreous hemorrhage: Ultrasound biomicro-scopy findings. Ophthalmology. 2004; 111:1215–21.
17. Yeh PT, Yang CM, Yang CH, Huang JS. Cryotherapy of the anterior retina and sclerotomy sites in diabetic vitrectomy to prevent recurrent vitreous hemorrhage. An ultrasound biomicro-scopy study. Ophthalmology. 2005; 112:2095–102.
Go to : 
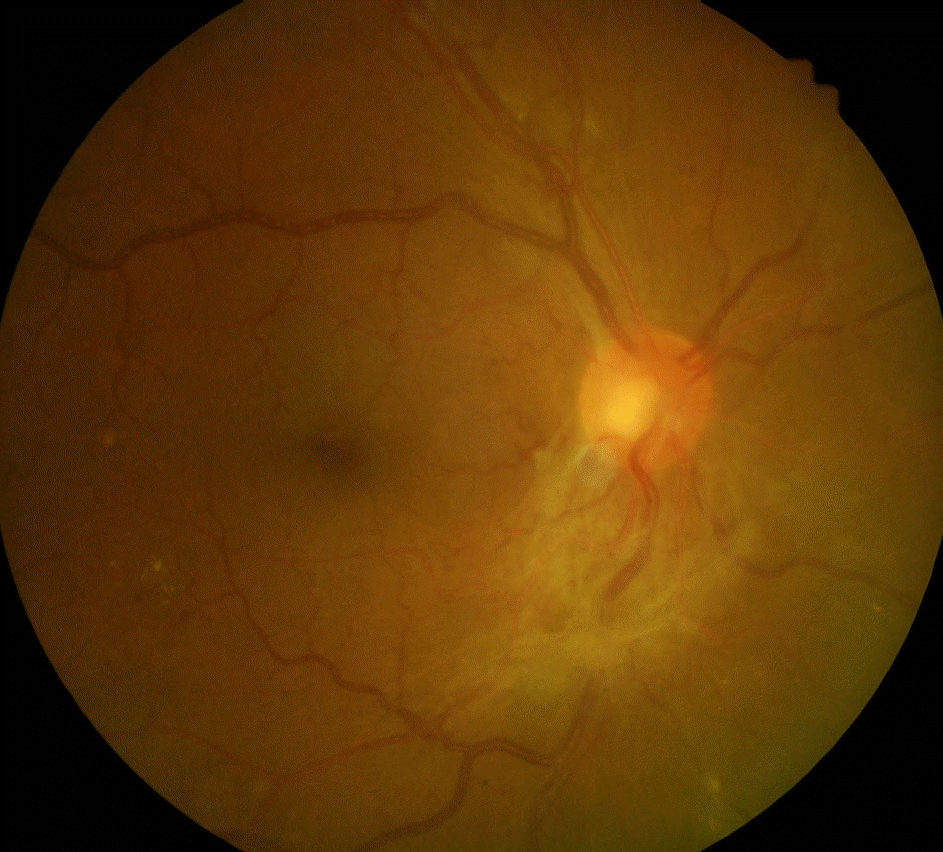 | Figure 1.Preoperative color fundus photography of one patient in group 1. Initial presentation shows extensive fibrovacular tractional membrane overlying the optic disc. |
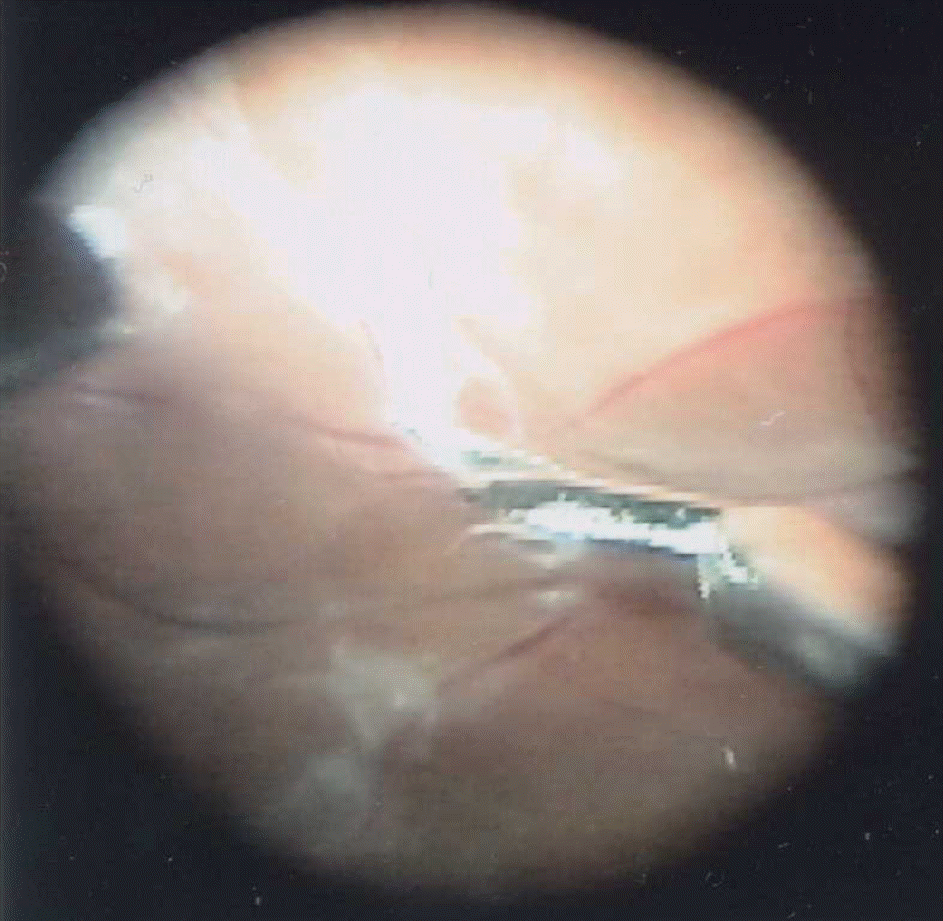 | Figure 2.Intraoperative fundus photography of one patient in group 1. Delamination of fibrovascular membrane with vertical scissors from the retina was technically easier than by conventional vitrectomy without preoperative intravitreal bevacizumab injection. |
Table 1.
Preoperative characteristics of each group
| Group 1 bevacizumab (+) | bevacizumab (−) Group 2 | p value | |
|---|---|---|---|
| No. of eyes | 30 | 30 | |
| No. of Patient (male) | 30 (20) | 29 (16) | |
| Mean age (year) | 55.07±11.9 | 52.43±8.6 | 0.330∗ |
| Duration of diabetes (year) | 14.33±6.6 | 11.90±8.0 | 0.207∗ |
| Duration of follow up (month) | 13.43±2.27 | 14.10±8.48 | 0.854∗ |
| Type 1/Type 2 DM | 1/29 | 1/28 | 0.954† |
| HbA1c (%) | 7.82±1.60 | 8.12±2.21 | 0.576† |
| Chronic renal failure (%) | 0.347† | ||
| Absent | 22 (73.3%) | 25 (83.3%) | |
| Present | 8 (26.7%) | 5 (16.7%) | |
| Hypertension (%) | 0.602† | ||
| Absent | 12 (40%) | 14 (46.7%) | |
| Present | 18 (60%) | 16 (56.3%) | |
| Preoperative PRP (%) | 0.638† | ||
| Grade 0 | 5 (16.7%) | 7 (23.3%) | |
| Grade 1 | 8 (26.6%) | 7 (23.3%) | |
| Grade 2 | 17 (56.7%) | 16 (53.4%) | |
| Lens status | 0.559† | ||
| Phakic | 21 (70%) | 23 (76.7%) | |
| Pseudophakic | 9 (30%) | 7 (23.3%) | |
| Surgical indication | |||
| Progressive fibrovascular proliferation | 1 (3.3%) | 0 (0%) | |
| Tractional retinal detachment | 6 (20.0%) | 9 (30%) | |
| Vitreous hemorrhage | 23 (76.7%) | 21 (70%) |
Table 2.
Comparison of the preoperative and postoperative BCVA∗
| Group 1 bevacizumab (+) | Group 2 bevacizumab (−) | p value† | |
|---|---|---|---|
| Preoperative BCVA (logMAR) | 1.28±0.75 | 1.57±0.95 | 0.913 |
| Postoperative BCVA (logMAR) | 0.44±0.46 | 0.53±0.33 |
Table 3.
Complications in each group, number of eyes (%)
| Group 1 bevacizumab (+) | Group 2 bevacizumab (−) | p value∗ | |
|---|---|---|---|
| Intraoperative bleeding | 14 (46.7%) | 11 (36.7%) | 0.3 |
| Postoperative bleeding | 4 (13.3%) | 14 (46.7%) | 0.005 |
| Transient ocular hypertension | 7 (23.3%) | 9 (32.1%) | 0.324 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download