Abstract
Purpose:
To compare the effect of an intravitreal injection of triamcinolone acetonide with bevacizumab in the treatment of diabetic macular edema (DME).
Methods:
For this study, the medical records of patients with diabetic macular edema, who received intravitreal triamcinolone injection (IVTA) or intravitreal bevacizumab injection (IVB), were reviewed. Best corrected visual acuity (BCVA), central macular thickness (CMT) and total macular volume (TMV) were evaluated before injection and at 1 week, 1 month, 2 months, 3 months, and 6 months after injection. The adverse events, such as increased intraocular pressure, and progression of cataract, were also reviewed.
Results:
A total of 72 eyes from 72 patients, (IVTA: 40 eyes, IVB: 32 eyes) were included in this study. In the IVTA group, BCVA improved significantly at 1 week after injection and was maintained until 3 months after injection. In the IVB group, BCVA improved significantly at 1 week after injection and was maintained until 2 months after injection. In the IVTA group, CMT and TMV decreased significantly at 1 week after injection and were maintained until 3 months after injection, while in the IVB group CMT and TMV were maintained until 2 months after injection. The IVTA group showed significantly better results in visual improvement, CMT and TMV reduction compared to the results of the IVB group, from 1 month to 3 months after injection. In the IVTA group, intraocular pressure increased to more than 25 mmHg in 12.5% of patients during the follow-up period.
Go to : 
References
1. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. IV. Diabetic macular edema. Ophthalmology. 1984; 91:1464–74.
2. Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985; 103:1796–806.
3. Ryan SJ. Nonproliferative diabetic retinopathy. Chew EY, Ferris FL III, editors. Retina. 4th ed.New York: Mosby, Elsevier Inc.;2006. 2:chap. 67.
4. Wilson C, Berkowitz BA, Sato Y, et al. Treatment with intravitreal steroid reduces Blood-retinal barrier breakdown due to retinal photocoagulation. Arch Ophthalmol. 1992; 110:1155–9.

5. Funatsu H, Yamashita H, Noma H, et al. Increased levels of vascular endothelial Growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002; 133:70–7.

6. Antcliff RJ, Marshall J. The pathogenesis of edema in diabetic maculopathy. Semin Ophthalmol. 1999; 14:223–32.

7. Fischer S, Renz D, Schaper W, Karliczek GF. In vitro effects of dexamethasone on hypoxia− induced hyperpermeability and expression of vascular endothelial growth factor. Eur J Phamacol. 2001; 411:231–43.
8. Nauck M, Karakiulakis G, Perruchoud AP, et al. Corticosteroids inhibit the expression of vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Phamacol. 1998; 341:309–15.
9. Larsson J, Zhu M, Sutter F, et al. Relation between reduction of foveal thickness and visual acuity in diabetic macular edema treated with intravitreal triamcinolone. Am J Ophthalmol. 2005; 139:802–6.

10. Selim Kocabora M, Kucuksahin H, Gulkilik G, et al. Treatment of diabetic macular edema with intravitreal triamcinolone acetonide injection: functional and anatomical outcomes. J Fr Ophthalmol. 2007; 30:32–8.
11. Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002; 109:920–7.

12. Tano Y, Chandler D, Machemer R. Treatment of intraocular proliferation with intravitreal injection of triamcinolone acetonide. Am J Ophthalmol. 1980; 90:810–6.

13. Jonas JB, Kreissig I, Sofker A, et al. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol. 2003; 121:57–61.

14. Sutter FK, Simpson JM, Grilles MC. Intravitreal triamcinolone for diabetic edema that persists after laser treatment: three month efficacy and safety results of a prospective, randomized, double− masked, placebo-controlled clinical trial. Ophthalmology. 2004; 111:2044–9.
15. Massin P, Andren F, Haouchine B, et al. Intravitreal triamcinolone acetonide for diabetic macular edema-Preliminary results of a prospective controlled trial. Ophthalmology. 2004; 111:218–24.
16. Verma LK, Vivek MB, Kumar A, et al. A prospective controlled trial to evaluate the adjunctive role of posterior subtenon triamcinolone in the treatment of diffuse diabetic macular edema. J Ocular Pharmacol Ther. 2004; 20:277–84.

17. Funatsu H, Yamashita H, Ikeda T, et al. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2003; 110:1690–6.

18. Funstsu H, Yamashita H, Sakata K, et al. Vitreous levels of vascular endothelial growth factor and intracellular adhesion molecule 1 are related to diabetic macular edema. Ophthalmology. 2005; 112:806–16.
19. Boulton M, Foreman D, Williams G, et al. VEGF localization in diabetic retinopathy. Br J Ophthalmol. 1998; 82:561–8.
20. Adamis AP, Miller JW, Bernal MT, et al. Increase vascular endothelial growth factor level in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994; 118:445–50.
21. Macugen Diabetic Retinopathy Study Group. A phase II rando-mized double-marked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macularedema. Ophthalmology. 2005; 112:1747–57.
22. Rosenfeld PJ, Fung AE, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005; 36:336–9.

23. Haritoglou C, Kook D, Neubauer A, et al. Intravitreal bevacizu-mab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006; 26:999–1005.

24. Hijikata K, Masuda K. Visual prognosis in Behet's disease: effects of cyclophosphamide and colchicines. Jpn J Ophthalmol. 1978; 22:506–19.
25. Strom C, Sander B, Klemp K, et al. Effect of ruboxistaurin on blood-retinal barrier permeability in relation to severity of leakage in diabetic macular edema. Invest Ophthalmol Vis Sci. 2005; 46:3855–8.
26. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004; 25:581–611.

27. Shimura M, Nakazawa T, Yasuda K, et al. Comparative therapy evaluation of intravitreal bevacizumab and triamcinolone acetonide on persistent diffuse diabetic macular edema. Am J Ophthalmol. 2008; 145:854–61.

28. Arevallo JF, Fromow-Guerra J, Quiroz-Mercado H, et al. Primary bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month flollow-up. Ophthalmology. 2007; 114:743–50.
29. Paccola L, Costa RA, Folgosa MS, et al. Intravitreal triamcinolone versus bevacizumab for treatment of refractory diabetic macular edema (IBEME study). Br J Ophthalmol. 2008; 92:76–80.
30. Stefansson E. Ocular oxygenation and the treatment of diabetic retinopathy. Surv Ophthalmol. 2006; 51:364–80.
31. McCuen B II, Bessler M, Tano Y, et al. The lack of toxicity of intravitreally administered triamcinolone acetonide. Am J Ophthalmol. 1981; 91:785–8.
32. Moshfeghi D, Kaiser P, Scot I, et al. Acute endophthalmitis following Intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003; 136:791–6.

33. Nelson M, Tennant M, Sivalingam A, et al. Infectious and pre-sumed noninfectious endophthalmitis after intravitreal triamcinolone acetonide injection. Retina. 2003; 23:686–91.

34. Roth D, Chieh J, Spirn M, et al. Noninfectious endophthalmitis associated with intravitreal triamcinolone injection. Arch Ophthalmol. 2003; 121:1279–82.

35. Jonas JB, Spandau UH, Schlichtenbrede F. Short-term compli-cations of intravitreal injections of triamcinolone and bevacizumab. Eye. 2008; 22:590–1.

36. Pieramici DJ, Avery RL, Castellarin AA, et al. Case of anterior uveitis after intravitreal injection of bevacizumab. Retina. 2006; 26:841–2.

37. Meyer CH, Mennel S, Schmidt JC, Kroll P. Acute retinal pigment epithelial tear following intravitreal bevacizumab (Avastin) injection for occult choroidal neovascularization seconday to age related macular degeneration. Br J Ophthalmol. 2006; 90:1207–8.
Go to : 
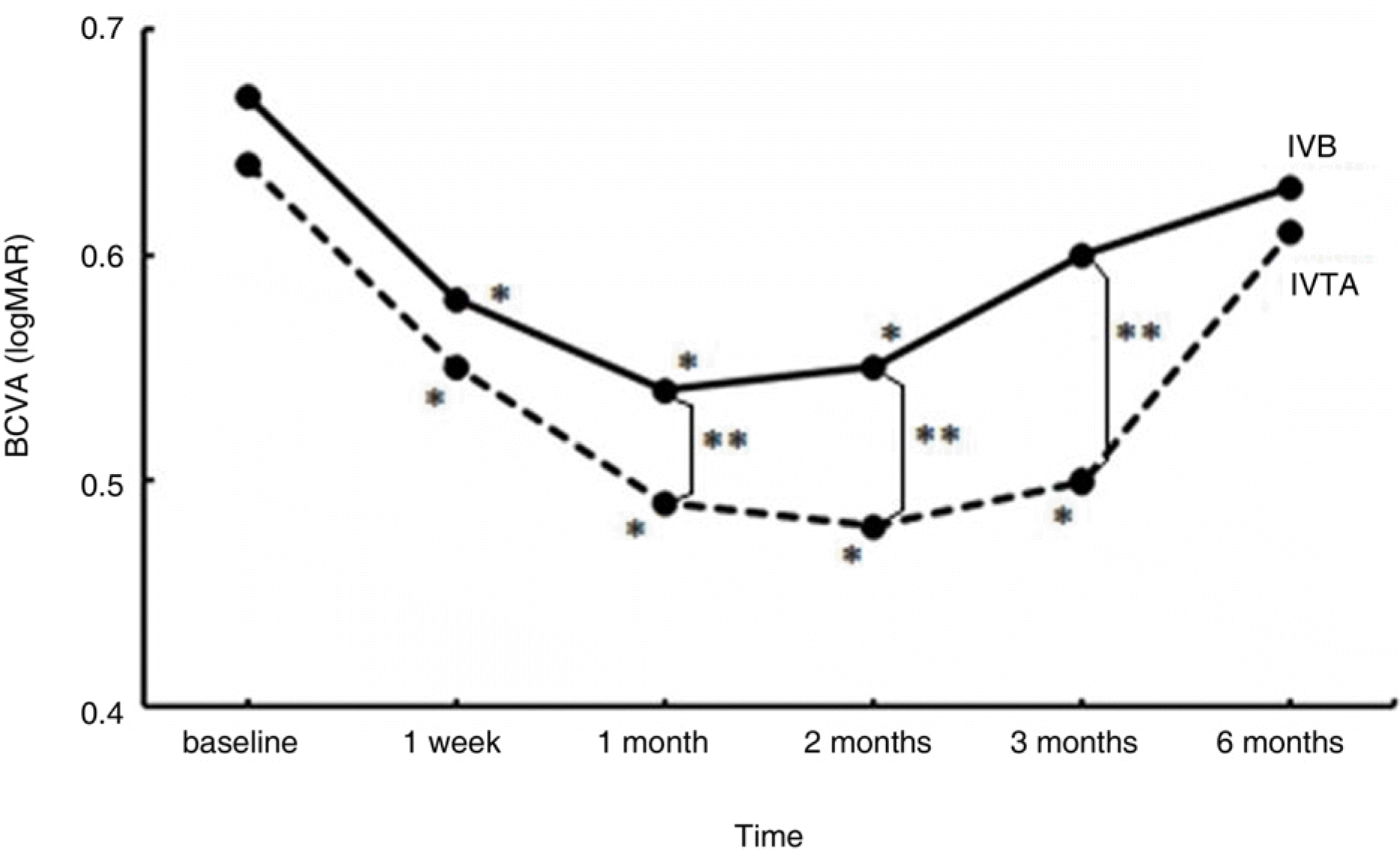 | Figure 1.Change in best corrected visual acuity (BCVA, logMAR) after intravitreal bevacizumab (IVB) and tri-amcinolone injections (IVTA)at follow-up visits. ∗ denotes statistically significant change from baseline at each visit within group (p<0.05). ∗∗ denotes statistically significant difference between two groups at each follow-up visit (p<0.05). |
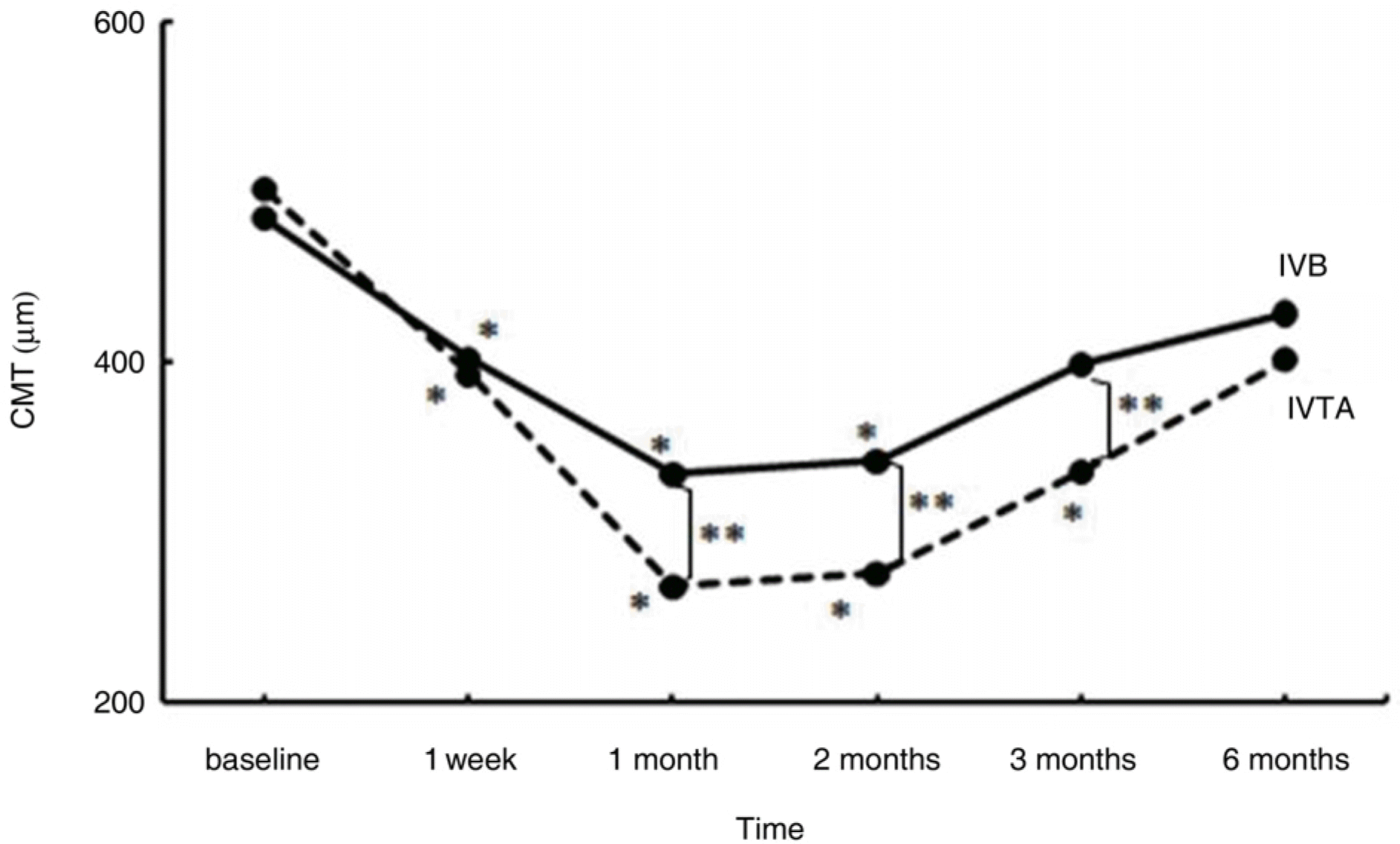 | Figure 2.Change in central macular thickness (CMT, µm) after intravitreal bevacizumab (IVB) and triamcinolone injections (IVTA) at follow-up visits. ∗ denotes statistically significant change from baseline at each visit within group (p<0.05). ∗∗ denotes statistically sig-nificant difference between two groups at each follow− up visit (p<0.05). |
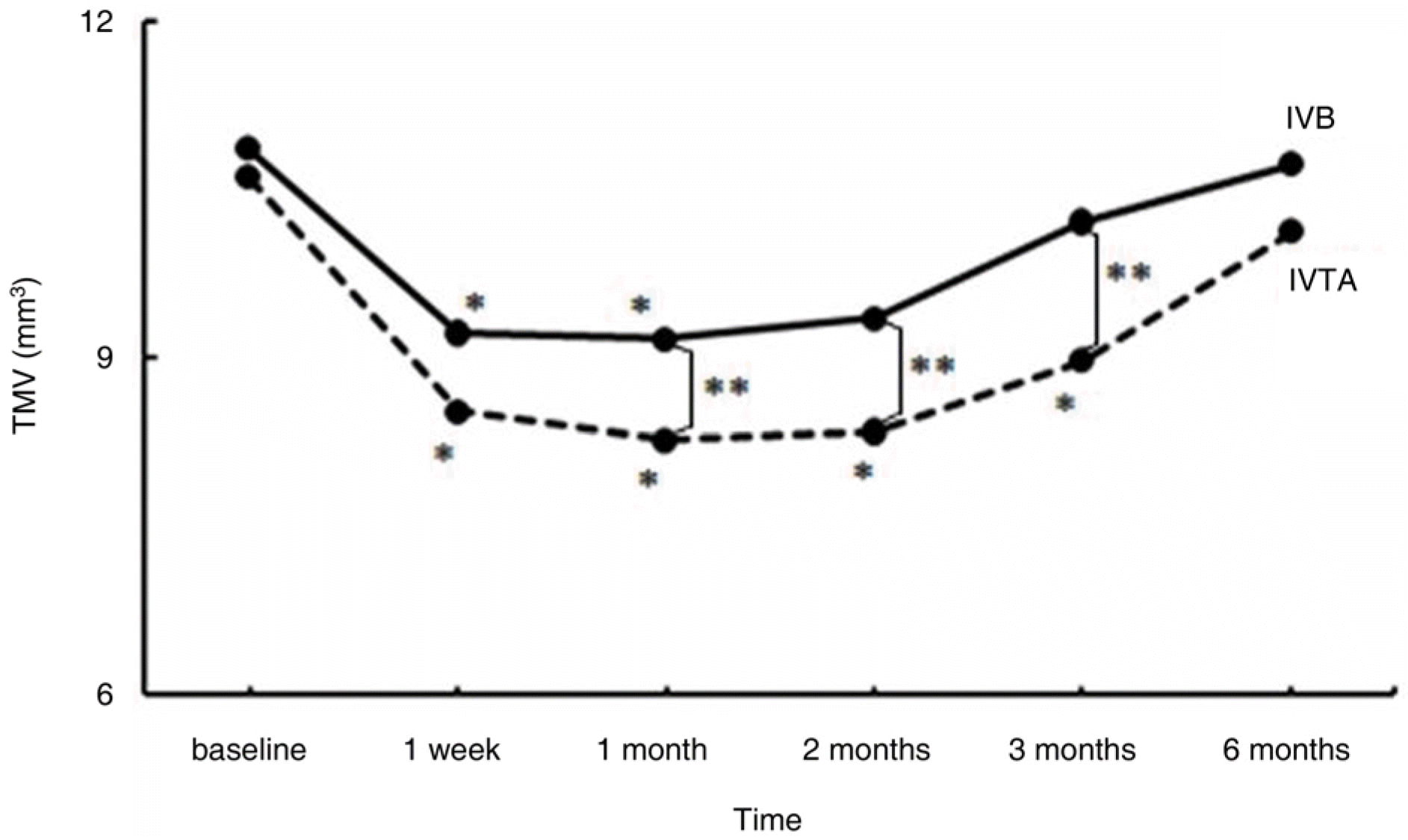 | Figure 3.Change in total macular volume (TMV, mm3) after intravitreal bevacizumab (IVB) and triamcinolone injections (IVTA) at follow-up visits. ∗ denotes sta-tistically significant change from baseline at each visit within-group (p<0.05). ∗∗ denotes statistically signi-ficant difference between two groups at each follow-up visit (p<0.05). |
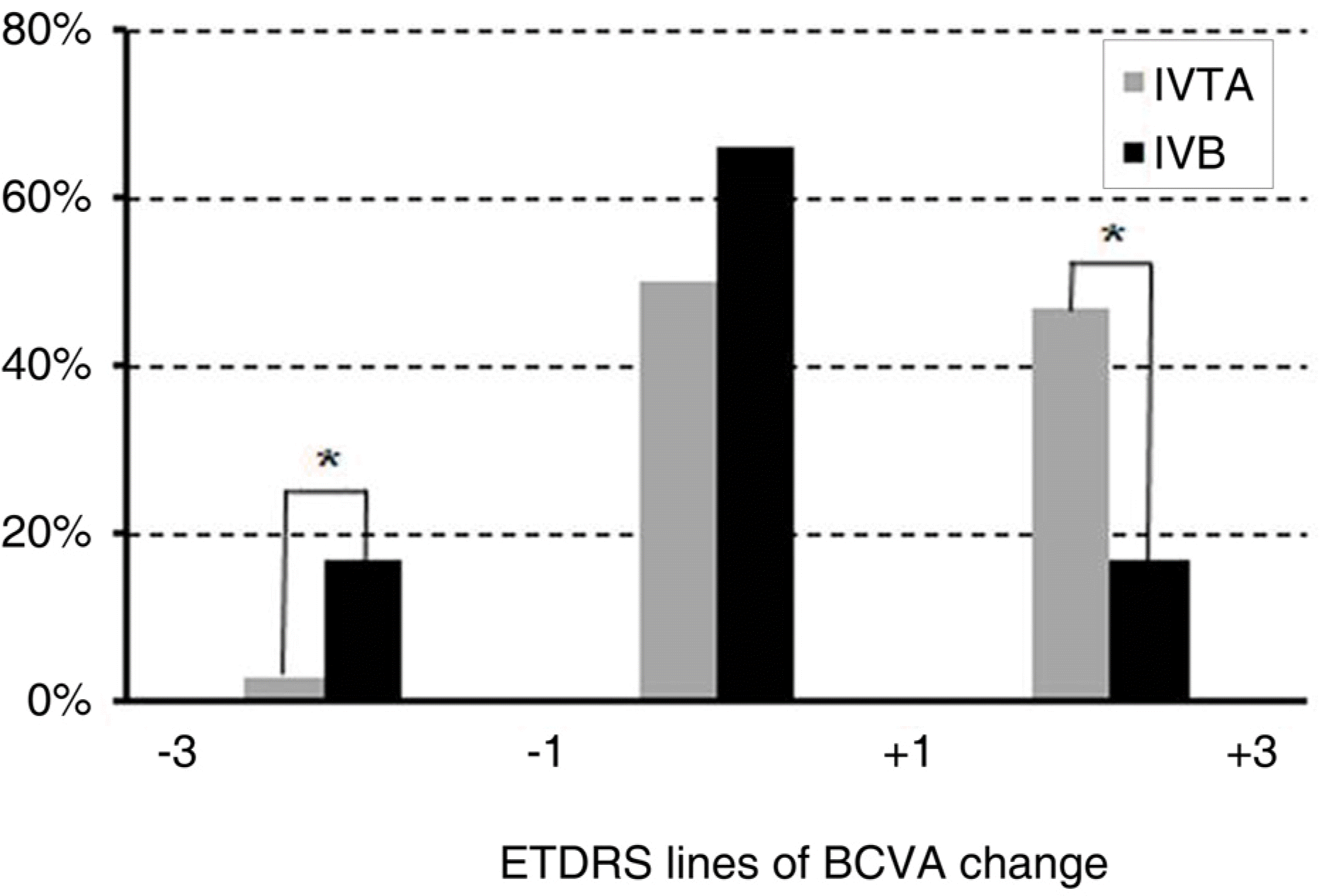 | Figure 4.The proportion of patients losing (−3 lines ~-1 line), maintaining (−1 line~+1 line), or gaining (+1~+3) best corrected visual acuity (BCVA) at 3 months. ∗ indicates a statistically significant difference between the two groups. |
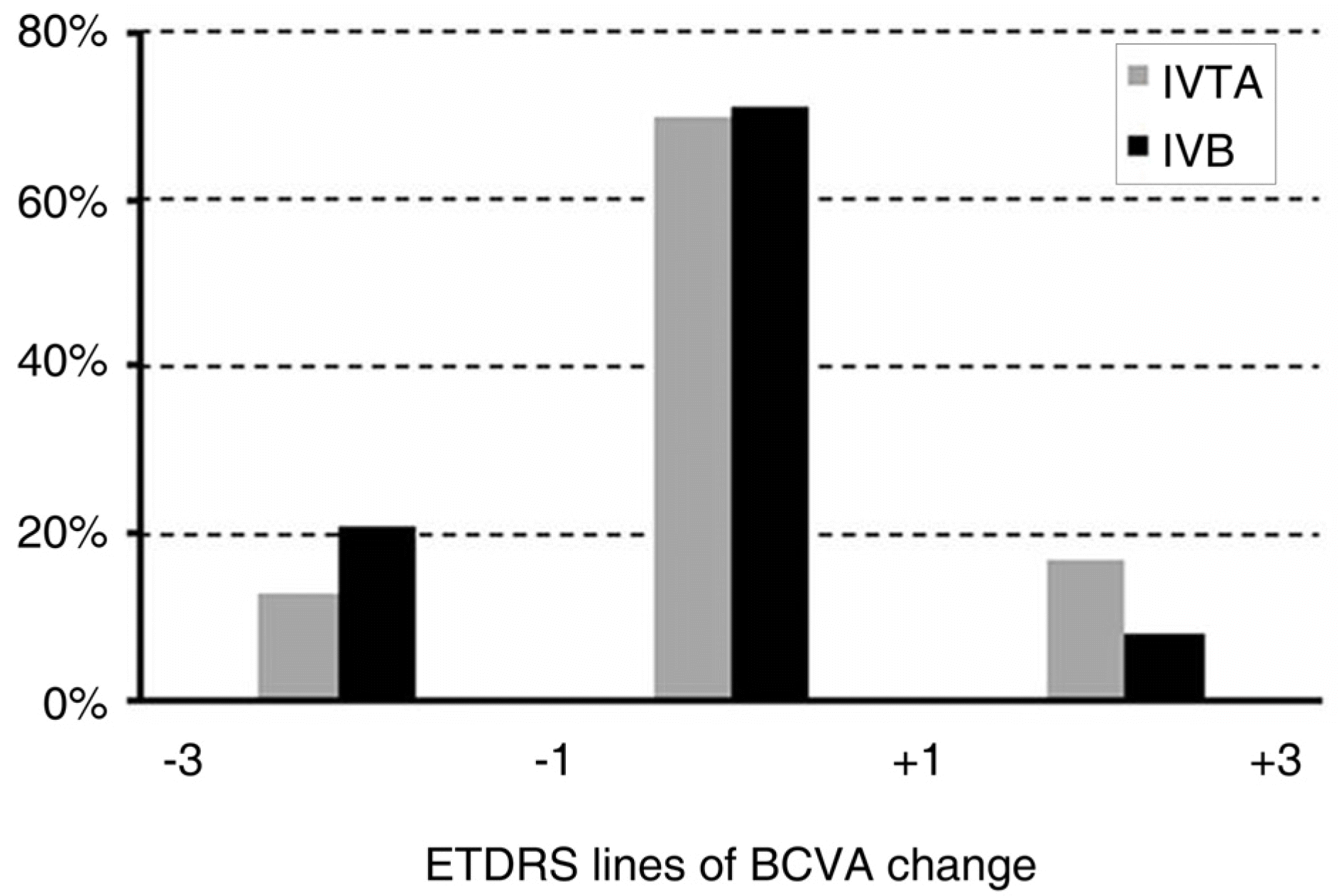 | Figure 5.The proportion of patients losing (−3 lines ~-1 line), maintaining (−1 line~+1 line), or gaining (+1~+3) best corrected visual acuity (BCVA) at 6 months. There was no statistically significant difference between the two groups. |
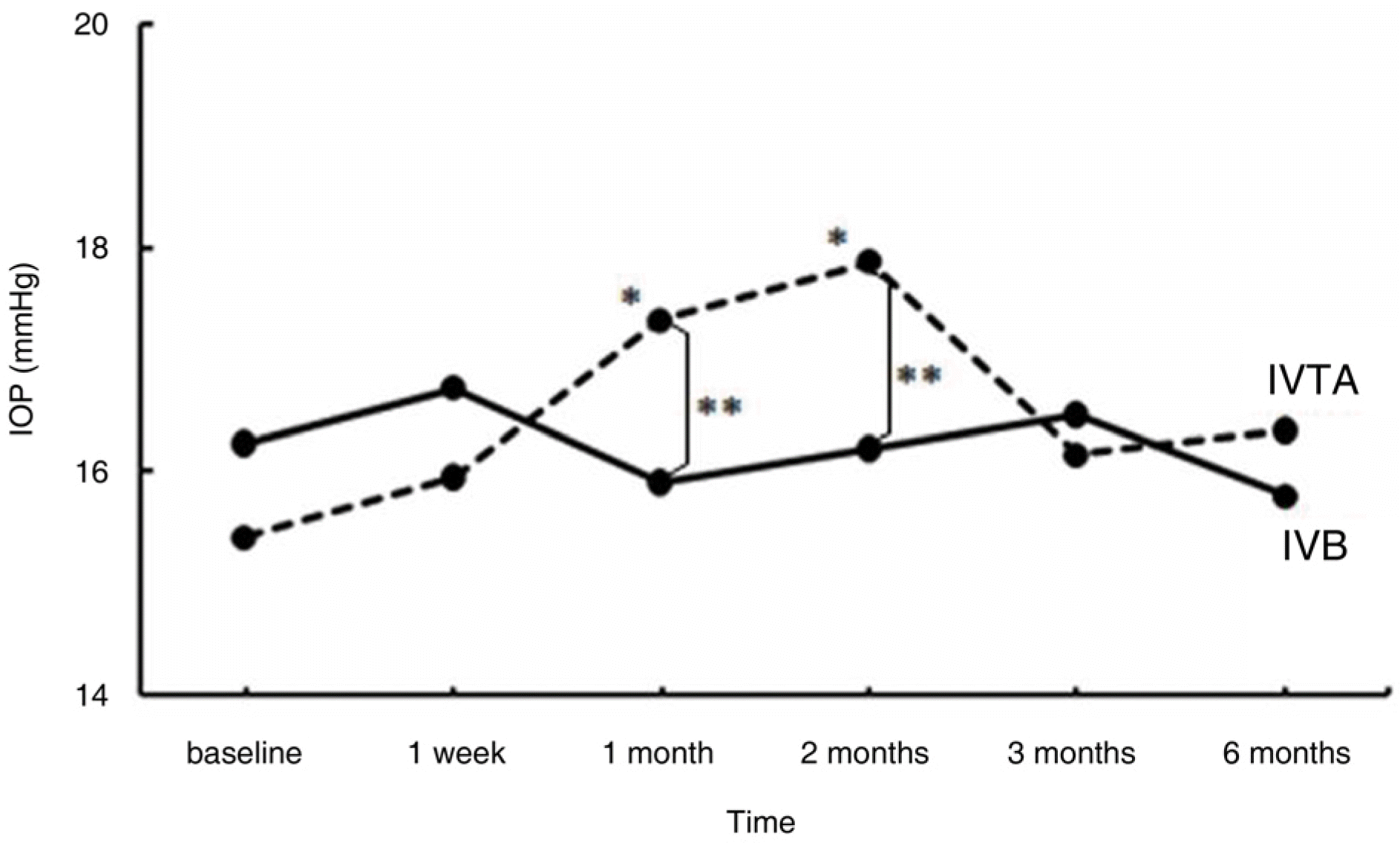 | Figure 6.Change in intraocular pressure (IOP, mm Hg) after intravitreal bevacizumab (IVB) and triamcinolone injections (IVTA) at follow-up visits. ∗ denotes statis-tically significant change from baseline at each visit within group (p<0.05). ∗∗ denotes statistically significant difference between the two groups at each visit (p<0.05). |
Table 1.
Baseline characteristics
| IVTA group | IVB group | p value | |
|---|---|---|---|
| Number of eyes | 40 | 32 | |
| Systemic | |||
| Age (years) | 59.2 | 61.1 | 0.317 |
| Female (%) | 37.5 | 40.6 | 0.215 |
| Duration of DM (years) | 13.07 | 12.81 | 0.271 |
| HbA1c (%) | 6.89 | 7.02 | 0.485 |
| Systolic BP (mmHg) | 142 | 144 | 0.571 |
| Diastolic BP (mmHg) | 92 | 88 | 0.436 |
| Ocular | |||
| BCVA∗ (logMAR) | 0.64 | 0.67 | 0.512 |
| IOP† (mmHg) | 15.41 | 16.25 | 0.516 |
| CMT‡ (µm) | 500.86 | 484.06 | 0.183 |
| Type of DME§ | 0.532 | ||
| Sponge | 22 | 15 | |
| Cystoid | 18 | 17 | |
| serous RD∏ | 0 | 0 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download