Abstract
Purpose
To evaluate the incidence, causative organism, clinical features, and visual outcomes of acute endophthalmitis following intravitreal injection.
Methods
For all intravitreal triamcinolone acetonide, bevacizumab, and lucentis injections performed in our outpatient clinic between January 2006 and June 2008, the number of injections, indications, type of administered drugs, and method of injection were investigated. The medical records of the patients with acute endoththalmitis were reviewed retrospectively.
Results
The total number of intravitreal injections was 10,153. The incidence of acute endophthalmitis for all intravitreal injections was 0.020% (2/10,153) with 0.030% (1/3,383) for the triamcinolone acetonide, 0.015% (1/6,552) for the bevacizumab, and 0.000% (0/218) for the ranibizumab drug injections. Streptococcus species were confirmed in the bacterial culture of two eyes with acute endotphthalmitis. After early vitrectomy and intravitreal antibiotics injection, one eye maintained vision but the other eye developed phthisis.
Go to : 
References
1. Ho J, Lowenstein JI. Endophthalmitis associated with intravitreal injection. Int Ophthalmol Clin. 2007; 47:199–208.
2. Jager RD, Aiello LP, Patel SC, et al. Risk of intravitreous injection: A comprehensive review. Retina. 2004; 24:676–98.
3. Aiello LP, Brucker AJ, Chang S, et al. Evolving guidelines for intravitreous injections. Retina. 2004; 24:S3–19.

4. Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003; 136:791–6.

5. Nelson ML, Tennant MT, Silvalignam A, et al. Infectious and presumed noninfectious endophthalmitis after intravitreal triamcinolone acetonide injection. Retina. 2003; 23:686–91.

6. Sohn HJ, Nam DH. Infectious endophthalmitis after intravitreal injection of triamcinolone acetonide. J Korean Ophthalmol Soc. 2006; 47:1865–70.
7. Gragoudas ES, Adamis AP, Cunnungham ET, et al. Pegatanib for neovascular age-related macular degeneration. N Engl J Med. 2004; 351:2805–16.
8. Macugen diabetic retinopathy study group. A phase II randomized double-masked trial of pegatanib, an antivascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005; 112:1747–57.
9. Heier Js, Antosyk AN, Pavam PR, et al. Ranibizumab for treatment of neovascular ange-related macular degeneration. Ophthalmology. 2006; 113:633–42.
10. Pilli S, Kotsolis A, Spaide RF, et al. Endophthalmitis associated with intravitreal antivascular endothelial growth factor therapy injections in an office setting. Am J Ophthalmol. 2008; 145:879–82.

11. Mason JO, White MF, Feist RM, et al. Incidence of acute onset endophthalmitis following intravitreal bevacizumab (Avastin) injection. Retina. 2008; 28:564–7.

12. Ta CN. Minimizing the risk of endophthalmitis following intravitreous injections. Retina. 2004; 24:699–705.

13. Apt L, Isenberg S, Yoshimori R, Khwarg S. Chemical preparation of the eye in ophthalmic surgery:effect of povidoneiodine on the conjunctiva. Arch Ophthalmol. 1984; 102:728–9.
14. Isenberg SJ, Apt L. Yoshimori R, Khwarg S. Chemical preparation of the eye in ophthalmic surgery: comparison of povidoneiodine on the conjunctiva with a prophylactic antibiotic. Arch Ophthalmol. 1985; 103:1340–2.
15. Safar A, Dellimore MC. The effect of povidone iodine flush versus drops on conjunctival colonization before intravitreal injections. Int Ophthalmol. 2007; 27:307–12.

16. Caro JJ, Ta CN, Ho H, et al. Bacterial contamination of ocular surface and needles in patients undergoing intravitreal injections. Retina. 2008; 28:877–83.
17. Speaker MG, Milch FA, Shah MK, et al. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology. 1991; 98:639–50.

18. Bucher RS, Hall E, Reed DM, et al. Effect of intravitreal triamcinolone acetonide on susceptibility to experimental bacterial endophthalmitis and subsequent response to treatment. Arch Ophthalmol. 2005; 123:649–53.

19. Scott IU, Flynn HW. The role of topical antibiotic prophylaxis for intravitreal injections. Arch Ophthalmol. 2007; 125:974–6.

20. Meyer CH, Mennel S, Eter N. Incidence of endophthalmitis after intravitreaal avastin injection with and without postoperative topical antibiotic application. Opthalmologe. 2007; 104:952–7.
21. Bannerman TL, Rhoden DL, McAllister SK, et al. The source of coagulase-negative staphylococci in the endophthalmitis vitrectomy study. A comparison of eyelid and intraocular isolates using pulsed-field gel electrophoresis. Arch Ophthalmol. 1997; 115:357–61.
Go to : 
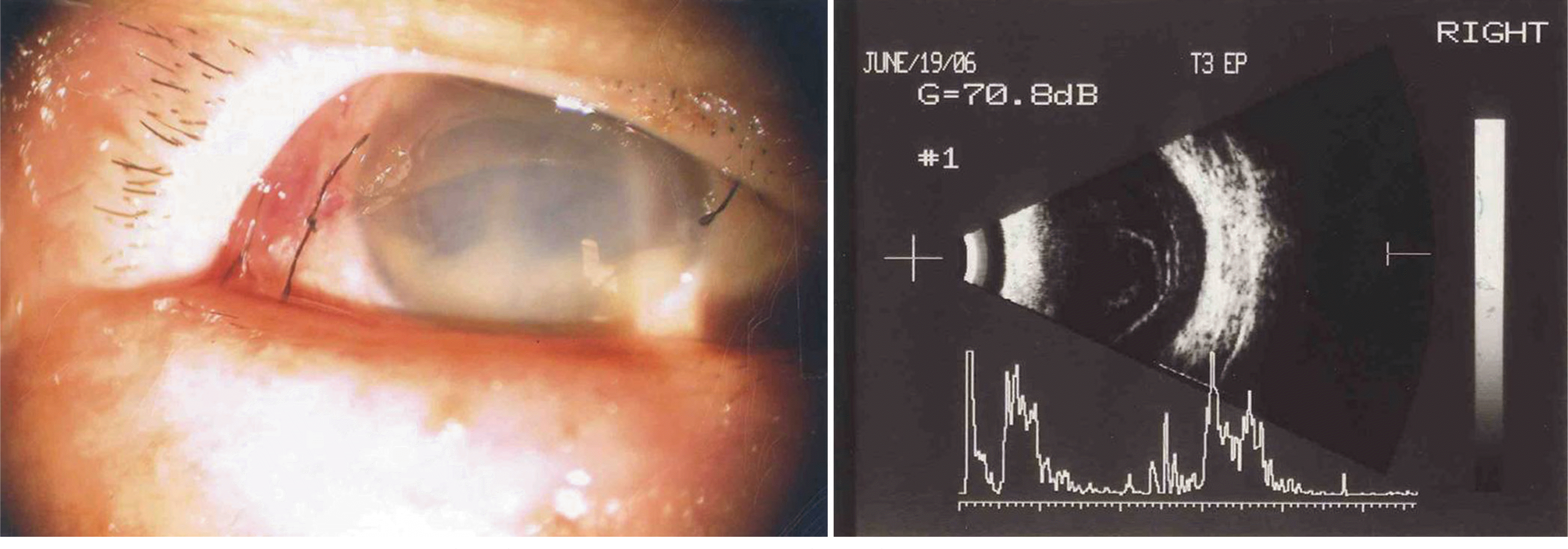 | Figure 2.Slit lamp photograph (left) and ultrasonogram (right) obtained 4 days after intravitreal triamcinolone acetonide injection. |
Table 1.
Patient demographics
| | | IVTA* | Avastin | Lucentis |
|---|---|---|---|---|
| | M:F | 1819: 1564 | 3720: 2832 | 119: 99 |
| | Age | 58.1± 13.2 | 59.3±13.2 | 59.8± 13.6 |
| | OD†:OS‡ | 1760: 1623 | 3430: 3122 | 103: 115 |
| | DMR§ | 1835 | 2818 | 0 |
| | CNV∏ | 417 | 2330 | 207 |
| | BRVO# | 640 | 615 | 0 |
| | CRVO** | 339 | 486 | 0 |
| Disease | CSC†† | 6 | 125 | 3 |
| | PCV‡‡ | 3 | 64 | 5 |
| | RAP§§ | 1 | 1 | 3 |
| | Others | 142 | 113 | 0 |
| | Total | 3383 | 6552 | 218 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download