Abstract
Purpose
To investigate the cellular protective effects of hypoxic preconditioning against oxidative stress in a staurosporine-differentiated RGC-5 cell line and the relevance of protein kinase C subtype expression.
Methods
The minimum staurosporine concentration and exposure time necessary to morphologically fully differentiate RGC-5 cells were determined. Cytotoxic injury was provided by oxidative stress with 800 µM hydrogen peroxide (H2 O2) for 15 hours to morphologically fully-differentiated cells. The cytoprotective effect of hypoxic preconditioning was found by exposing the cell line to 0.3% oxygen for different periods of time. Quantifiable changes in the expression of mRNAs and proteins of the isoenzymes α, β, γ, δ, ε, ζ of protein kinase C were determined before and after 1, 2, 15, and 24 hours of hypoxic preconditioning.
Results
Axonal growth in RGC-5 cells after the induction of differentiation with staurosporine caused these cells to resemble neurons. The minimal concentration and exposure time to staurosporine that evoked full differentiation of RGC-5 cells was exposure to 2 µM staurosporine for 1 hour. An LDH assay demonstrated that hypoxic preconditioning had neuroprotective effects against hydrogen peroxide-induced oxidative stress. Protein and mRNA levels of PKC isoforms α and ε increased after preconditioning.
Conclusions
Hypoxic preconditioning of staurosporine-differentiated RGC-5 cells had a cytoprotective effect against oxidative stress. The associated increase of mRNA and proteins of PKC isoenzymes α and ε suggest some functional relevance of these isoenzymes to the cytoprotective effects conferred by hypoxic preconditioning.
Go to : 
References
1. Goldberg I. Weinreb RN, Kitazawa J, Krieglestein GK, editors. How common is glaucoma worldwide? Glaucoma in the 21st Century. Landau, Germany: Mosby;2000:1–8.
2. Giammanco R, Dardanoni G, Ponte F.Prevalence of glaucoma in a population. Acta Ophthalmol Scand. 1995; 73:222–5.
3. Cedrone C, Culasso F, Cesareo M. . Prevalence of glaucoma in Ponza. Ophthalmic Epidemiol. 1997; 4:59–72.
4. Owsley C, McGwin G Jr, Ball K. Vision impairment, eye disease, and motor vehicle crashes in the elderly. Ophthalmic Epidemiol. 1998; 5:101–13.
5. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000; 130:429–40.
6. Collaborative Normal-Tension Glaucoma Study Group (CNTGSP). Comparison of glaucomatous progression between untreated patients with normal‐ tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998; 126:487–97.
8. Flammer J, Haefliger IO. Orgül S, Resink T. Vascular ysregulation: a principal risk factor for glaucoma damage? J Glaucoma. 1999; 8:212–9.
9. Dowden J, Corbett D. Ischemic preconditioning in 18 to 20 month‐ old gerbils long‐ term survival with functional outcome measures. Stroke. 1999; 30:1240–6.
10. Kristian T. Siesjö BK. Calcium in ischemic cell death. Stroke. 1998; 29:705–18.
12. Hawaleshka A, Jacobsohn E. Ischemic preconditioning: mechanisms and potential clinical applications (Review). Can J Anaesth. 1998; 45:670–82.
13. Kitagawa K, Matsumoto M, Tagaya M. . Ischemic tolerance phenomenon found in the brain. Brain Res. 1990; 528:21–4.

14. Paratt JR. Protection of the heart by ischemic preconditioning: mechanisms and possibilities for pharmacological expression. Trends Pharmacol Sci. 1994; 15:19–25.
15. Barone FC, White RF, Spera PA. . Ischemic preconditioning and brain tolerance. Stroke. 1998; 29:1937–51.
16. Zhu Y, Ohlemiller KK, McMahan BK. . Constitutive nitric oxide synthase activity is required to trigger ischemic tolerance in mouse retina. Exp Eye Res. 2006; 82:153–63.

17. Ozbay D, Ozden S, Muftuoglu S. . Protective effect of ischemic preconditioning on retinal ischemia‐ reperfusion injury in rats. Can J Ophthalmol. 2004; 39:727–32.
18. Sakamoto K, Yonoki Y, Kuwagata M. . Histological protection against ischemia‐ reperfusion injury by early ischemic preconditioning in rat retina. Brain Res. 2004; 1015:154–60.
19. Matsumoto S, Shamloo M, Matsumoto E. . Protein kinase C-gamma and calcium/calmodulin-dependent protein kinase II alpha are persistently translocated to cell membranes of the rat brain during and after middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2004; 24:54–61.
20. Speechly‐ Dick ME, Mocanu MM, Yellon DM, Protein kinase C.Its role in ischemic preconditioning in the rat. Circ Res. 1994; 75:586–90.
21. Piccoletti R, Bendinelli P, Arienti D. Bernelli-Zazzera A. State and activity of protein kinase C in postischemic reperfused liver. Exp Mol Pathol. 1992; 56:219–28.
22. Padanilam BJ. Induction and subcellular localization of protein kinase C isozymes following renal ischemia. Kidney Int. 2001; 59:1789–97.

23. Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu Rev Neurosci. 1994; 17:551–67.

24. Chen L, Hahn H, Wu G. . Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci U S A. 2001; 98:11114–9.
25. Whitlock NA, Agarwal N, Ma JX, Crosson CE. Hsp27 upregulation by HIF‐1 signaling offers protection against retinal ischemia in rats. Invest Ophthalmol Vis Sci. 2005; 46:1092–8.

26. Charles I, Khalyfa A, Kumar DM. . Serum deprivation induces apoptotic cell death of transformed rat retinal ganglion cells via mitochondrial signaling pathways. Invest Ophthalmol Vis Sci. 2005; 46:1330–8.

27. Maher P, Hanneken A. The molecular basis of oxidative stress-induced cell death in an immortalized retinal ganglion cell line. Invest Ophthalmol Vis Sci. 2005; 46:749–57.

28. Krishnamoorthy RR, Agarwal P, Prasanna G. . Characterization of a transformed rat retinal ganglion cell line. Brain Res Mol Brain Res. 2001; 86:1–12.
Go to : 
 | Figure 1.The assessment of optimal level and duration in staurosporine treatment for differentiation of RGC-5: The shape of RGC-5 cell changes from fibroblast-like shape to neuron-like shape showing axon and multiple synapse with treatment of staurosporine. Staurosporine 1.0 µg for 2 hours seemed to be the minimum duration and the level for the appropriate morphological differentiation. Staurosporine 0 µg: 1 hr (A), 2 hr (B), 6 hr (C), 24 hr (D); Staurosporine 0.5 µg: 1 hr (E), 2 hr (F), 6 hr (G), 24 hr (H); Staurosporine 1.0 µg: 1 hr (I), 2 hr (J), 6 hr (K), 24 hr (L); Staurosporine 2.0 µg: 1 hr (M), 2 hr (N), 6 hr (O), 24 hr (P). |
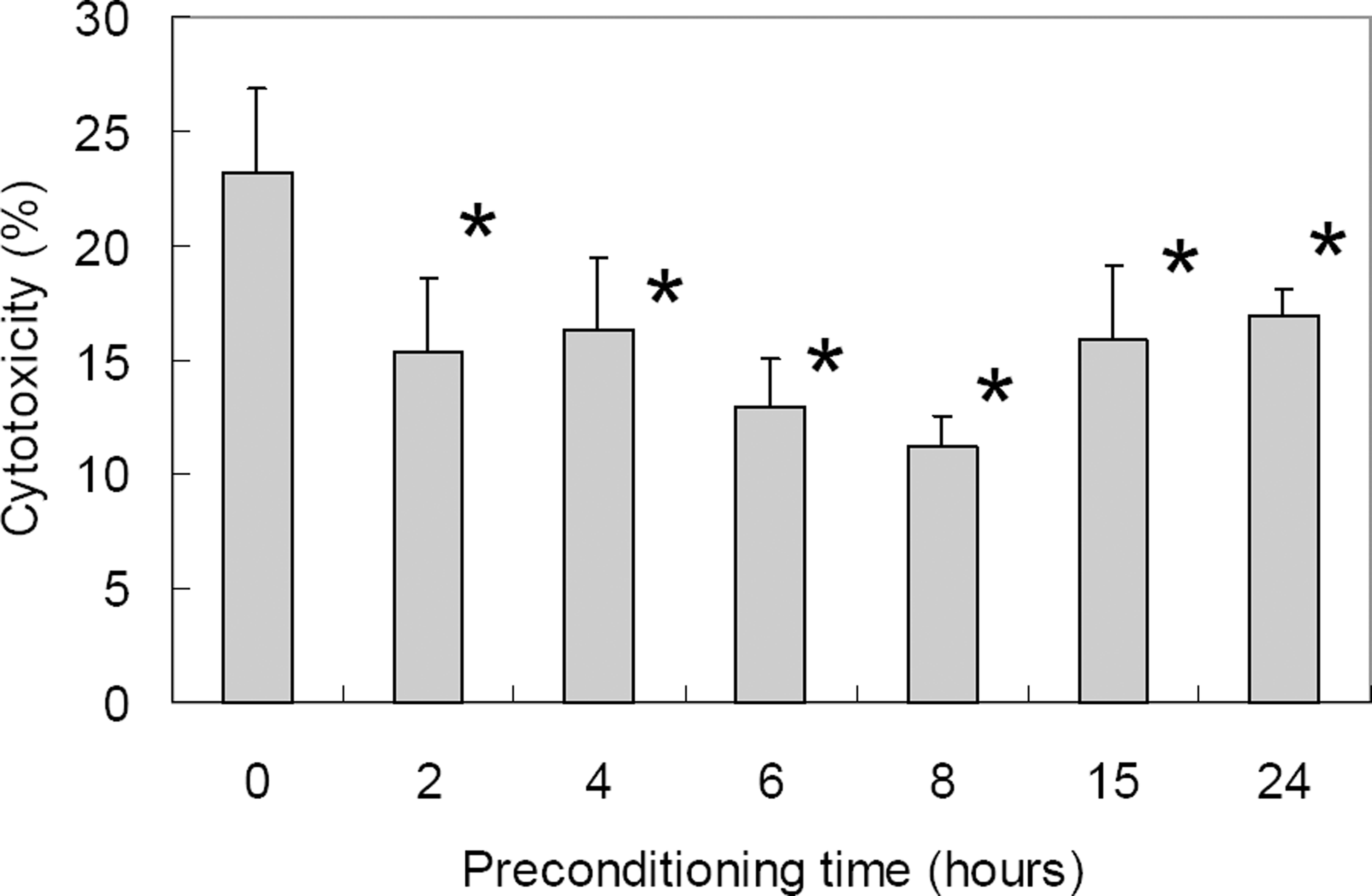 | Figure 2.Cytotoxicity of ischemic preconditioning (0.3% oxygen state) against oxidative stress (800 µM H2 O2 for 15 hr) in differentiated RGC-5 as demonstrated with LDH assay. With prolonged duration of ischemic preconditioning, RGC-5 survival increased up to 8 hrs of ischemic preconditioning after which cytotoxicity increased. Cytotoxicity after oxidative stress was statistically significantly decreased at 2 to 24 hours of ischemic preconditioning when compared to control (* p<0.05 by Mann-Whitney U test). |
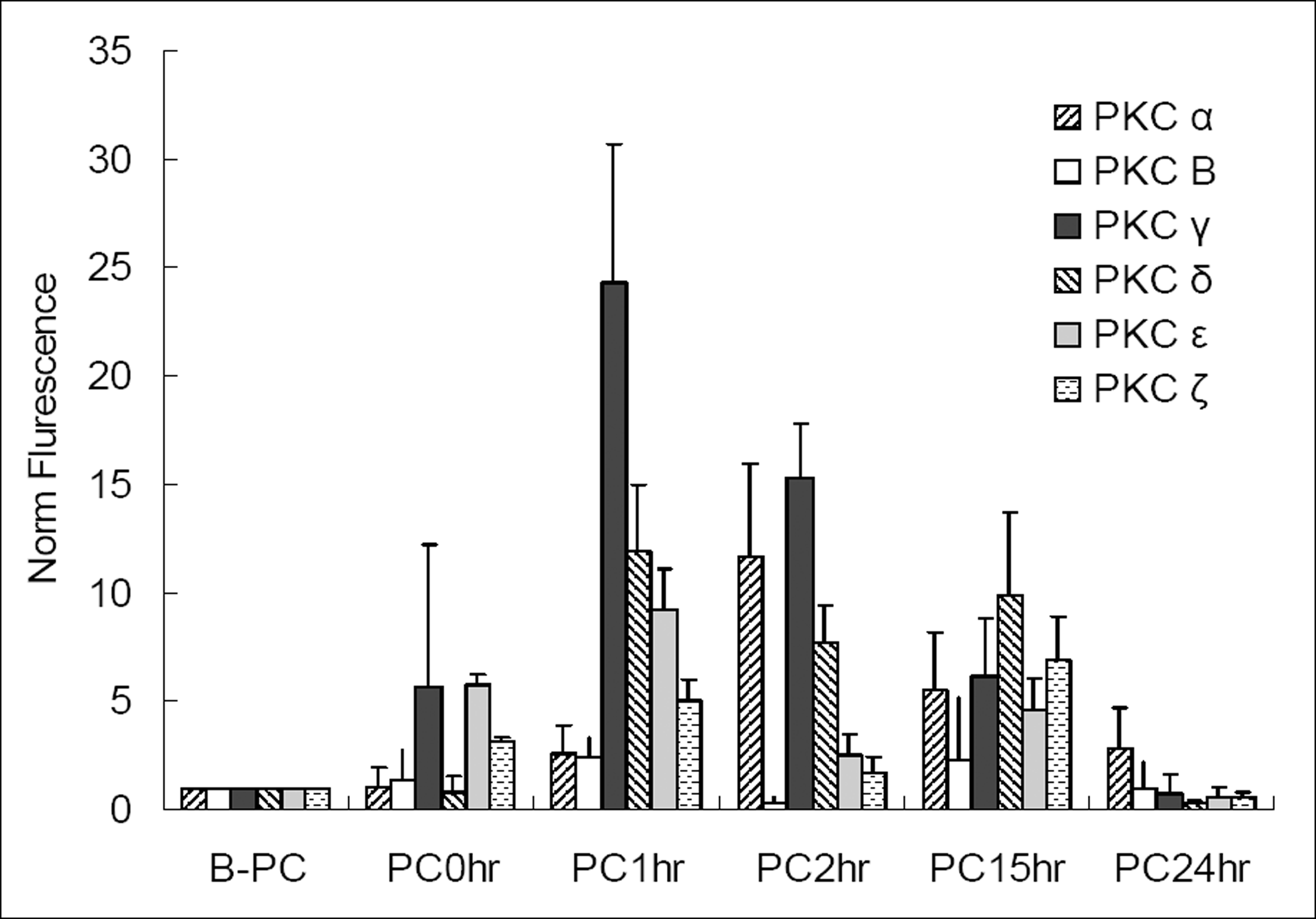 | Figure 3.Changes of PKC iso-enzyme mRNA before and after ischemic preconditioning (B-PC, before preconditioning; PC0hr, just after the preconditioning; PC1hr, 1 hour after preconditioning; PC2hr, 2 hours after preconditioning; PC15hr, 15 hours after preconditioning; PC24hr, 24 hours after preconditioning); We can see the elevation of level of PKC α, β, γ, δ, ε mRNA 1 or 2 hours after PC (preconditioning, 0.3% oxygen 8 hrs). The elevations are decreasing with time after PC. |
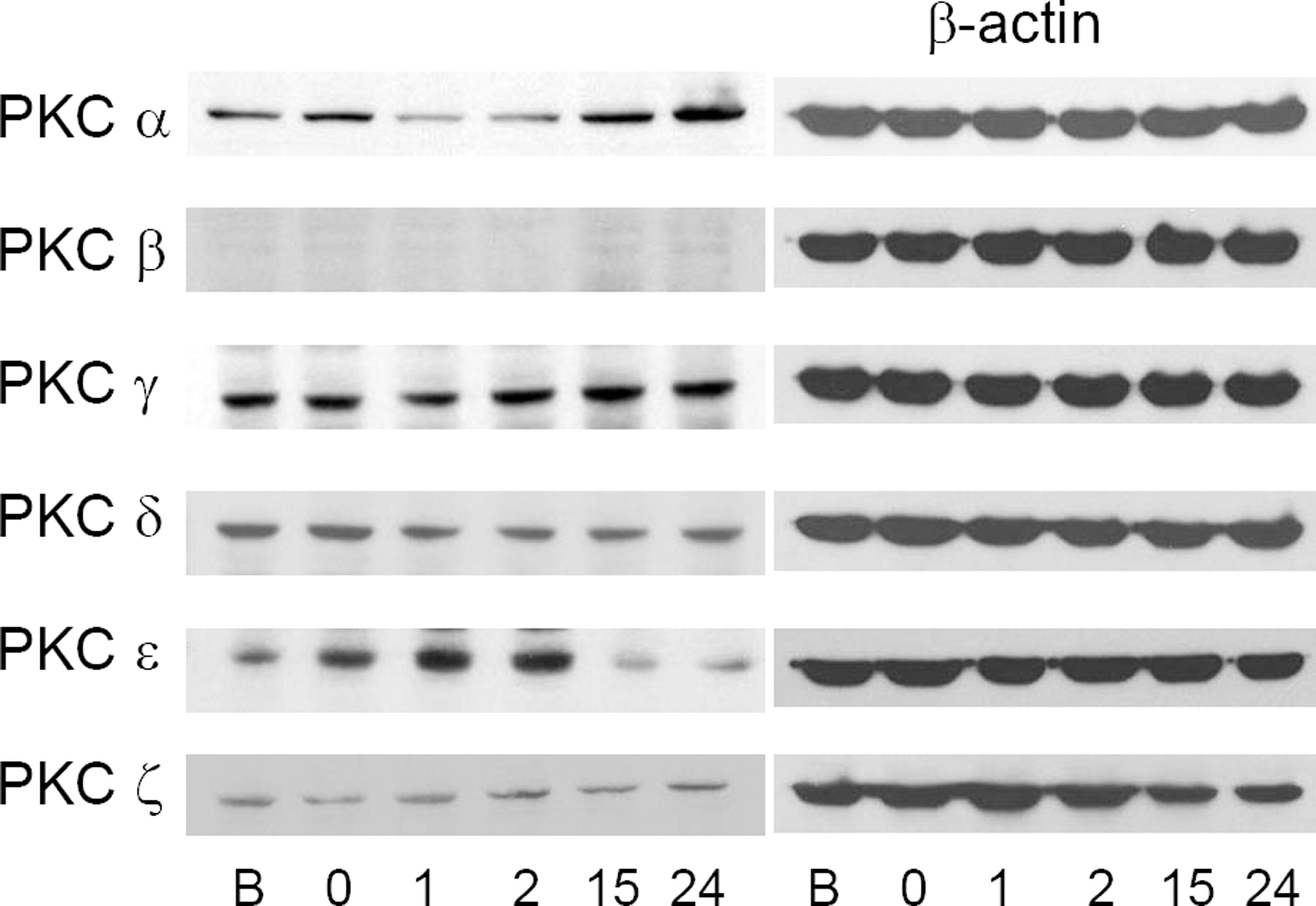 | Figure 4.Changes of PKC iso-enzyme protein before and after ischemic preconditioning (B, before preconditioning; 0, just after the preconditioning; 1, 1 hour after preconditioning; 2, 2 hours after preconditioning; 15, 15 hours after preconditioning; 24, 24 hours after preconditioning). PKC ε increased until 2 hours after the preconditioning, and PKC α increased from 15 hours after the preconditioning. These changes are relatively correlated with increasing tendency of mRNA. |
Table 1.
Primer sequences for real-time PCR (F=forward; R=reverse)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download