Abstract
Purpose
To retrospectively evaluate the clinical effect of intravitreal (IVT) and posterior subtenon (PSTT) triamcinolone acetonide (TA) injection in diabetic macular edema.
Methods
Fifty eyes of 43 patients with diabetic macular edema (DME) were included. Twenty-two eyes in the IVT group received 4 mg/0.1 ml intravitreal TA injections and 26 eyes in the PSTT group received 40 mg/1.0 ml subtenon TA injections. LogMAR visual acuity and intraocular pressure (IOP) were measured and, using OCT, central macular thickness (CMT) and total macular volume (TMV) were also measured before and 1, 3, and 6 months postoperatively.
Results
Both groups showed significant decreases in the mean CMT and TMV. The mean CMT in the IVT group was significantly thinner than that of the PSTT group 1 month postoperatively. The percentage reduction in CMT and TMV were also greater in the IVT group than the PSTT group 1 month postoperatively. Both groups showed significant improvements in mean visual acuity, with no significant difference between the two groups. However, at 1-month postoperative improvement in visual acuity was significantly greater in the IVT group than the PSTT group. The mean IOP in the IVT group was also greater than that in the PSTT 1 month postoperatively.
Go to : 
References
1. Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985; 103:1796–806.
2. Early Treatment Diabetic Retinopathy Study Research Group. Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 2. Ophthalmology. 1987; 94:761–74.
3. Early Treatment Diabetic Retinopathy Study Research Group. Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report no. 19. Arch Ophthalmol. 1995; 113:1144–55.
4. Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002; 109:920–7.

5. Jonas JB, Kreissig I, Sofker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol. 2003; 121:57–61.

6. Massin P, Aurden F, Haouchine B, et al. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology. 2004; 111:218–24.
7. Aurden F, Tod M, Massin P, et al. Pharmacokinetic- pharmacodynamic modeling of the effect of triamcinolone acetonide on central macular thickness in patients with diabetic macular edema. Invest Ophthalmol Vis Sci. 2004; 45:3435–41.
8. Sutter FK, Simpson JM, Gillies MC. Intravitreal triamcinolone for diabetic macular edema that persists after laser treatment: three-month efficacy and safety results of a prospective, randomized, double-masked, placebo-controlled clinical trial. Ophthalmology. 2004; 111:2044–9.
9. Kim IC, Cho NC, Ahn M. Intravitreal Injection of Triamcinolone for Refractory Diabetic Macular Edema. J Korean Ophthalmol Soc. 2004; 45:228–36.
10. Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003; 136:791–6.

11. Benz MS, Murray TG, Dubovy SR, et al. Endophthalmitis caused by Mycobacterium chelonae abscessus after intravitreal injection of triamcinolone. Arch Ophthalmol. 2003; 121:271–3.

12. Nelson ML, Tennant MT, Sivalingam A, et al. Infectious and presumed noninfectious endophthalmitis after intravitreal triamcinolone acetonide injection. Retina. 2003; 23:686–91.

13. Park HJ, Park JM, Oum BS. Presumed Noninfectious Endophthalmitis after Intravitreal Injection of Triamcinolone Acetonide. J Korean Ophthalmol Soc. 2005; 46:1419–23.
14. Jonas JB, Sofker A, Degenring R. Intravitreal triamcinolone acetonide as an additional tool in pars plana vitrectomy for proliferative diabetic retinopathy. Eur J Ophthalmol. 2003; 13:468–73.

15. Jonas JB, Kreissig I, Degenring R. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol. 2003; 87:24–7.

16. Kaushik S, Gupta V, Gupta A, et al. Intractable glaucoma following intravitreal triamcinolone in central retinal vein occlusion. Am J Ophthalmol. 2004; 137:758–60.

17. Melberg NS, Olk RJ. Corticosteroid-induced ocular hypertension in the treatment of aphakic or pseudophakic cystoid macular edema. Ophthalmology. 1993; 100:164–7.

18. Helm CJ, Holland GN. The effects of posterior subtenon injection of triamcinolone acetonide in patients with intermediate uveitis. Am J Ophthalmol. 1995; 120:55–64.

19. Tanner V, Kanski JJ, Frith PA. Posterior sub-Tenon’s triamcinolone injections in the treatment of uveitis. Eye. 1998; 12:679–85.

20. Okada AA, Wakabayashi T, Morimura Y, et al. Trans-Tenon’s retrobulbar triamcinolone infusion for the treatment of uveitis. Br J Ophthalmol. 2003; 87:968–71.

21. Cardillo JA, Melo LA Jr, Costa RA, et al. Comparison of intravitreal versus posterior sub-Tenon’s capsule injection of triamcinolone acetonide for diffuse diabetic macular edema. Ophthalmology. 2005; 112:1557–63.

22. Bakri SJ, Kaiser PK. Posterior subtenon triamcinolone acetonide for refractory diabetic macular edema. Am J Ophthalmol. 2005; 139:290–4.

23. Tunc M, Onder HI, Kaya M. Posterior sub-Tenon’s capsule triamcinolone injection combined with focal laser photocoagulation for diabetic macular edema. Ophthalmology. 2005; 112:1086–91.

24. Hayashi K, Hayashi H. Intravitreal versus retrobulbar injections of triamcinolone for macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2005; 139:972–82.

25. Schindler RH, Chandler D, Thresher R, Machemer R. The clearance of intravitreal triamcinolone acetonide. Am J Ophthalmol. 1982; 93:415–7.

26. Machemer R. Five cases in which a depot steroid (hydrocortisone acetate and methylprednisolone acetate) was injected into the eye. Retina. 1996; 16:166–7.
27. Challa JK, Gillies MC, Penfold PL, et al. Exudative macular degeneration and intravitreal triamcinolone: 18 month follow up. Aust N Z J Ophthalmol. 1998; 26:277–81.

28. Danis RP, Ciulla TA, Pratt LM, Anliker W. Intravitreal triamcinolone acetonide in exudative age-related macular degeneration. Retina. 2000; 20:244–50.

29. Greenberg PB, Martidis A, Rogers AH, et al. Intravitreal triamcinolone acetonide for macular oedema due to central retinal vein occlusion. Br J Ophthalmol. 2002; 86:247–8.

30. Antcliff RJ, Spalton DJ, Stanford MR, et al. Intravitreal triamcinolone for uveitic cystoid macular edema: an optical coherence tomography study. Ophthalmology. 2001; 108:765–72.

31. Young S, Larkin G, Branley M, Lightman S. Safety and efficacy of intravitreal triamcinolone for cystoid macular oedema in uveitis. Clin Experiment Ophthalmol. 2001; 29:2–6.

32. Benhamou N, Massin P, Haouchine B, et al. Intravitreal triamcinolone for refractory pseudophakic macular edema. Am J Ophthalmol. 2003; 135:246–9.

33. Ciulla TA, Walker JD, Fong DS, et al. Corticosteroids in posterior segment disease: An update on new delivery systems and new indications. Curr Opin Ophthalmol. 2004; 15:211–20.

34. McCuen BW 2nd, Bessler M, Tano Y, et al. The lack of toxicity of intravitreally administered triamcinolone acetonide. Am J Ophthalmol. 1981; 91:785–8.

35. Bakri SJ, Beer PM. The effect of intravitreal triamcinolone acetonide on intraocular pressure. Ophthalmic Surg Lasers Imaging. 2003; 34:386–90.

36. Jonas JB, Degenring RF, Kreissig I, et al. Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Ophthalmology. 2005; 112:593–8.

37. Yang YH, Kim KR, Yang SW, Yim HB. The Effect of Intravitreal Triamcinolone Acetonide on Intraocular Pressure. J Korean Ophthalmol Soc. 2004; 45:1081–5.
38. Gillies MC, Simpson JM, Billson FA, et al. Safety of an intravitreal injection of triamcinolone: results from a randomized clinical trial. Arch Ophthalmol. 2004; 122:336–40.
39. Pitkanen L, Ranta VP, Moilanen H, Urtti A. Permeability of retinal pigment epithelium: effects of permeant molecular weight and lipophilicity. Invest Ophthalmol Vis Sci. 2005; 46:641–6.
40. Ambati J, Gragoudas ES, Miller JW, et al. Transscleral delivery of bioactive protein to the choroid and retina. Invest Ophthalmol Vis Sci. 2000; 41:1186–91.
41. Nozik RA. Periocular injection of steroids. Trans Am Acad Ophthalmol Otolaryngol. 1972; 76:695–705.
42. Kalina PH, Erie JC, Rosenbaum L. Biochemical quantification of triamcinolone in subconjunctival depots. Arch Ophthalmol. 1995; 113:867–9.

43. Beer PM, Bakri SJ, Singh RJ, et al. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology. 2003; 110:681–6.

44. Mueller AJ, Jian G, Banker AS, et al. The effect of deep posterior subtenon injection of corticosteroids on intraocular pressure. Am J Ophthalmol. 1998; 125:158–63.

45. Smithen LM, Ober MD, Maranan L, Spaide RF. Intravitreal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol. 2004; 138:740–3.

46. Abel AD, Carlson JA, Bakri S, Meyer DR. Sclerosing lipogranuloma of the orbit after periocular steroid injection. Ophthalmology. 2003; 110:1841–5.
Go to : 
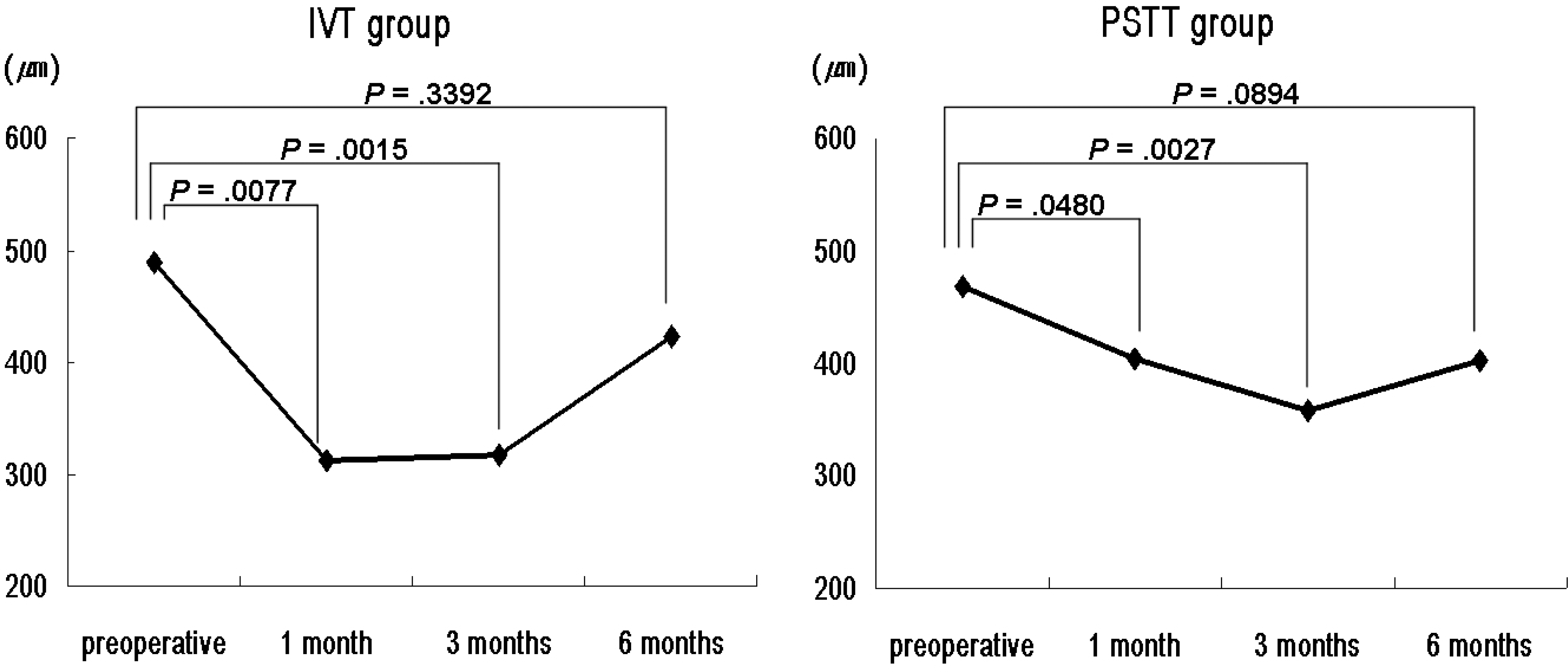 | Figure 1.Temporal changes of mean central macular thickness (CMT) in intravitreal TA injection (IVT) and subtenon TA injection (PSTT) groups. |
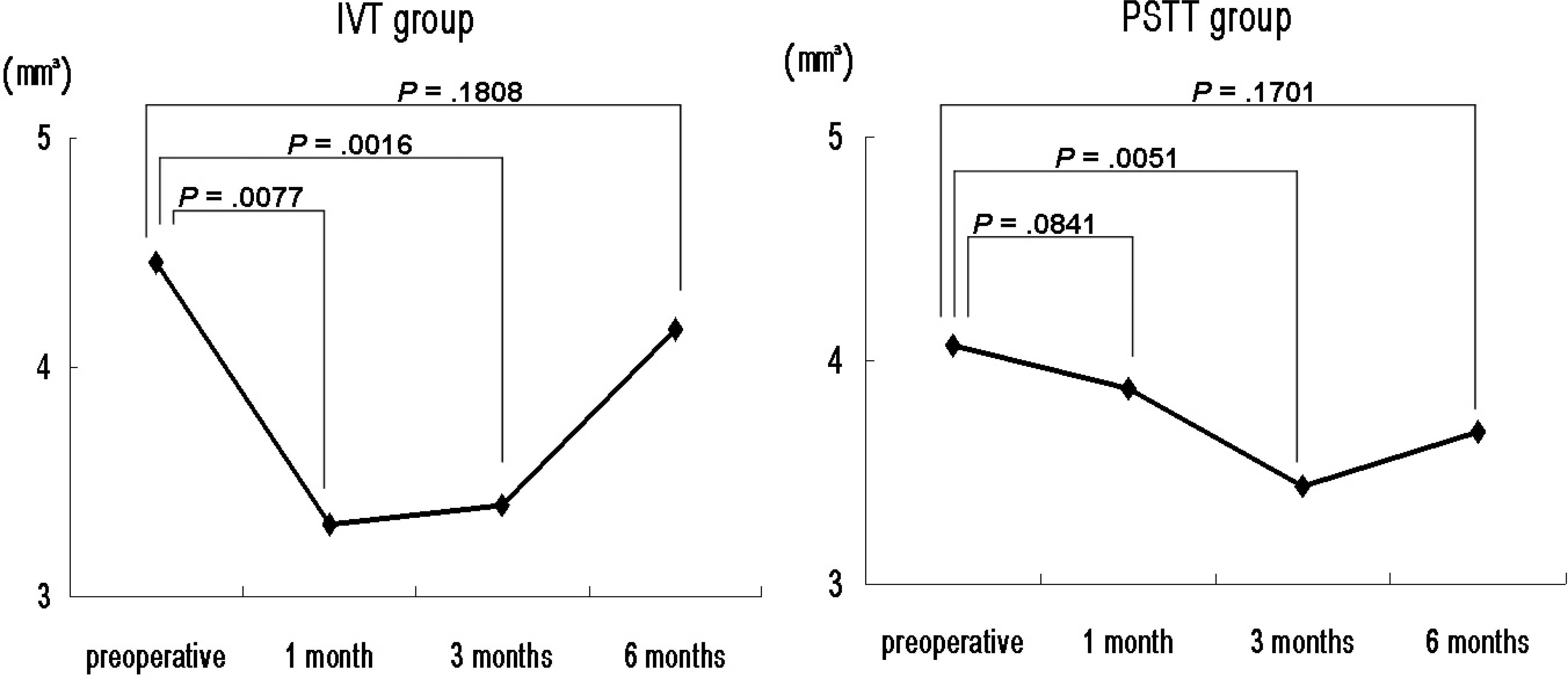 | Figure 2.Temporal changes of mean total macular volume (TMV) in intravitreal TA injection (IVT) and subtenon TA injection (PSTT) groups. |
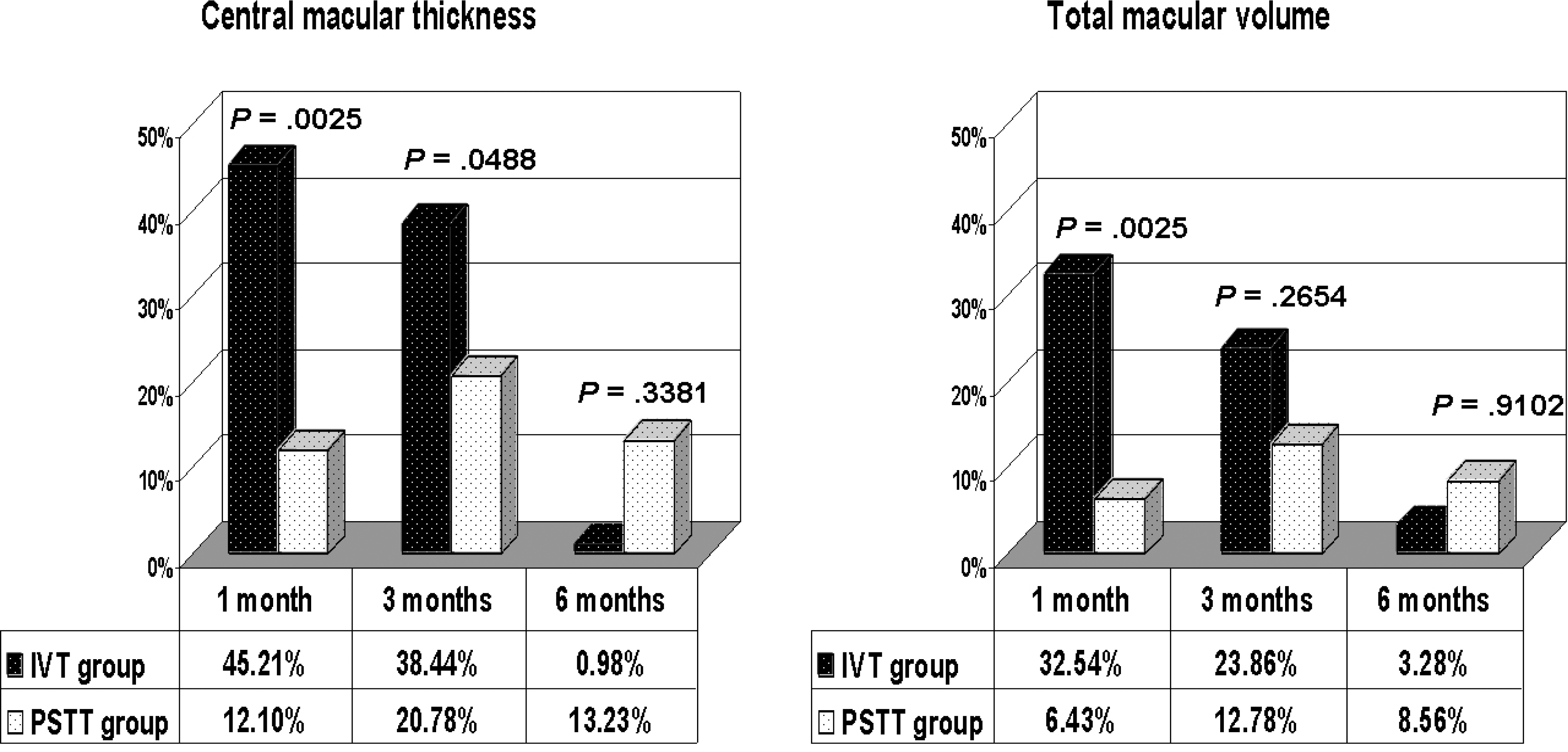 | Figure 3.Comparison of mean percentage reduction in central macular thickness and total macular volume between intravitreal TA injection (IVT) and subtenon TA injection (PSTT) groups. |
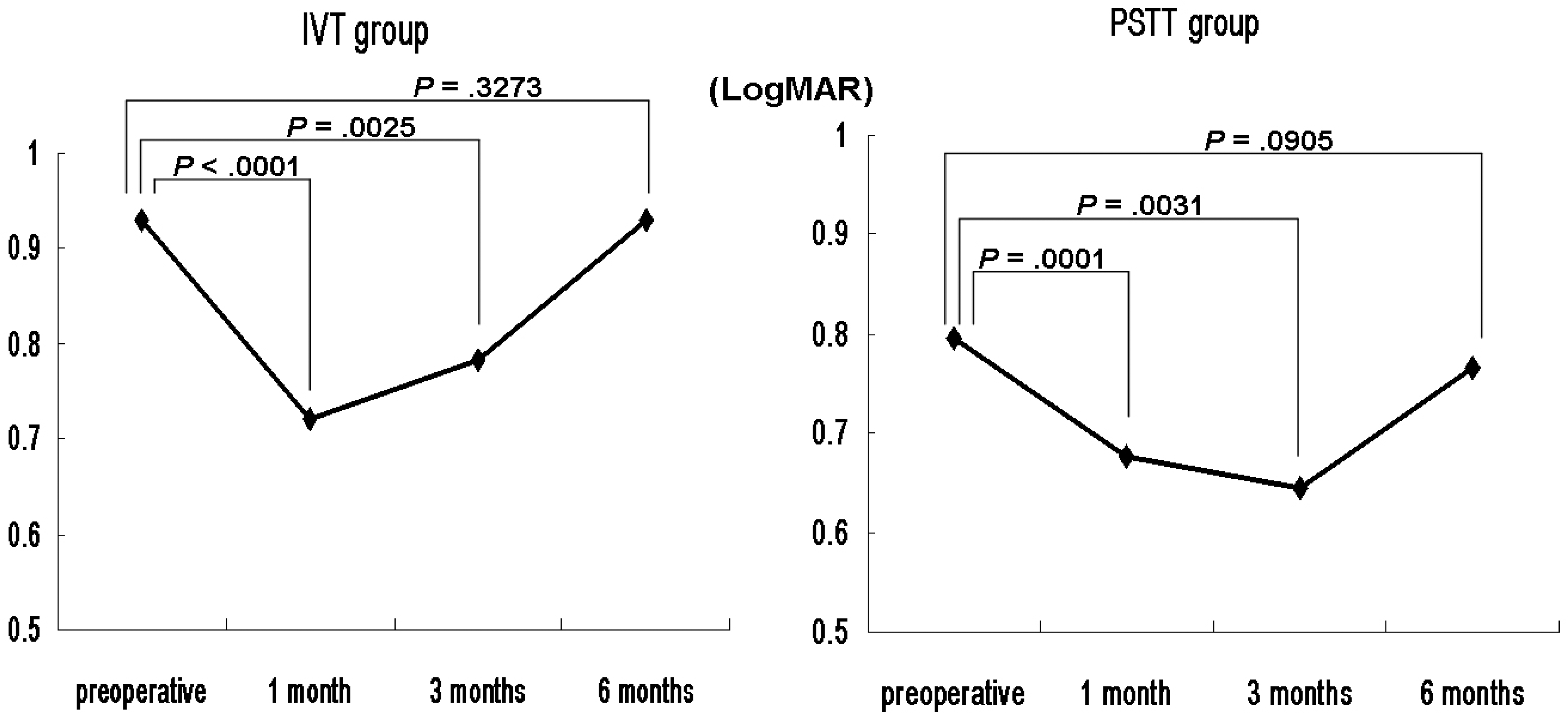 | Figure 4.Temporal changes of mean visual acuity (logMAR) in intravitreal TA injection (IVT) and subtenon TA injection (PSTT) groups. |
Table 1.
| Characteristics | IVT* group | PSTT† Group | p value |
|---|---|---|---|
| No. of eyes | 24 | 26 | |
| No. of patients (male) | 23 | 25 | |
| Age (years) | 58.17±8.51 | 59.65±7.93 | 0.566 |
| Male (female) | 10 (14) | 10 (16) | 0.817 |
| Left eye (right) | 11 (13) | 16 (10) | 0.266 |
| Duration of diabetes (years) | 13.21±7.34 | 13.08±6.54 | 0.884 |
| Hypertension | 6 | 8 | 0.65 |
| Previous PRP‡ | 0.23 | ||
| no treatment | 7 | 8 | |
| post-PRP‡ state | 15 | 14 | |
| PRP‡ during study | 2 | 4 | |
| Previous FLP§ | 2 | 1 | 0.211 |
| Lens status | 0.119 | ||
| Phakic | 17 | 23 | |
| pseudophakic | 7 | 3 | |
| ETDRS∏ grade | 0.729 | ||
| PDR# | 15 | 15 | |
| NPDR** | 9 | 11 |
Table 2.
| IVT* group | PSTT† group | p value | |
|---|---|---|---|
| CMT (µm) | |||
| Preoperative | 497.3±206.8 | 466.3±126.1 | 0.79 |
| Post-op 1 month | 305.7±141.3 | 409.3±128.3 | 0.038† |
| 3 months | 295.8±132.8 | 354.3±138.4 | 0.12 |
| 6 months | 441.3±130.5 | 401.2±143.0 | 0.38 |
| TMV (mm3) | |||
| Preoperative | 4.46±1.32 | 4.07±0.77 | 0.38 |
| Post-op 1 month | 3.32±0.83 | 3.87±0.96 | 0.15 |
| 3 months | 3.40±0.77 | 3.44±0.91 | 0.8 |
| 6 months | 4.17±1.16 | 3.68±0.96 | 0.19 |
Table 3.
| IVT* group | PSTT† group | p value | |
|---|---|---|---|
| Visual acuity | |||
| Preoperative | 0.929±0.299 | 0.796±0.321 | 0.106 |
| Post-op 1 month | 0.721±0.301 | 0.677±0.313 | 0.799 |
| 3 months | 0.783±0.350 | 0.644±0.347 | 0.223 |
| 6 months | 0.929±0.366 | 0.765±0.310 | 0.313 |
| Improvement in visual acuity | |||
| Post-op 1 month | -0.208 | -0.119 | 0.02§ |
| 3 months | -0.161 | -0.16 | 0.913 |
| 6 months | -0.043 | -0.106 | 0.452 |
Table 4.
| IVT* group | PSTT† group | p value | |
|---|---|---|---|
| IOP‡(mmHg) | |||
| Preoperative | 17.7±2.8 | 18.4±2.8 | 0.23 |
| Post-op 1 day | 17.5±3.3 | 18.3±2.4 | 0.3 |
| 1 week | 18.5±4.0 | 18.9±2.7 | 0.95 |
| 1 month | 21.7±5.1 | 18.8±3.2 | 0.045§ |
| 3 months | 20.3±3.7 | 19.0±2.8 | 0.27 |
| 6 months | 19.6±3.7 | 18.8±2.8 | 0.76 |
| Percentage increase in IOP‡(%) | |||
| Post-op 1 day | -0.3±17.6 | -1.9±17.6 | 0.652 |
| 1 week | 7.7±22.0 | 4.8±16.6 | 0.623 |
| 1 month | 24.7±34.1 | 3.8±15.7 | 0.023§ |
| 3 months | 17.5±22.8 | 2.8±13.4 | 0.048§ |
| 6 months | 12.9±17.9 | 2.0±13.6 | 0.082 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download