Abstract
Purpose
The purpose of this study was to investigate the prophylactic effect of brimonidine 0.15% on intraocular pressure (IOP) elevation and the risk factors for its elevation after intravitreal triamcinolone acetonide injection (IVTA).
Methods
A prospective, randomized clinical trial was conducted on 67 eyes of 64 patients undergoing IVTA. Eyes were randomly divided into two groups, those which had used brimonidine 0.15% (40 eyes) and those which had not used it (27 eyes). IOP was measured preoperatively, at one week, and monthly until six months postinjection in each group.
Results
The mean post injection IOP at one week was 11.93±3.36 mmHg for the group that had used brimonidine and 13.58±3.25 mmHg for the group that had not used it. The difference between the two groups was statistically significant at one week (p=0.049), but others were not statistically significant. An elevation in the IOP of more than 22 mmHg was seen in 8 eyes (20%) in the group using brimonidine and in 7 eyes (25.9%) in the group not using brimonidine. There was no difference in the incidence of IOP elevation between the two groups.
Conclusions
Prophylactic use of brimonidine 0.15% will prevent sudden IOP elevation and will, therefore, prevent damage to the retina and optic nerve. However, in the long term, there is no prophylactic effect of brimonidine 0.15% on IOP elevation because there was no difference in the incidence of IOP elevation of more than 22 mmHg between the two groups.
Go to : 
References
1. Antcliff RJ, Spalton DJ, Stanford MR, et al. Intravitreal triamcinolone for uveitic cystoid macular edema: an optical coherence tomography study. Ophthalmology. 2001; 108:765–72.

2. Jonas JB, Hayler JK, Sofker A, et al. Intravitreal injection of crystalline cortisone as adjunctive treatment of proliferative diabetic retinopathy. Am J Ophthalmol. 2001; 131:468–71.

3. Danis RP, Ciullar TA, Pratt LM, et al. Intravitreal triamcinolone acetonide in exudative age relative macular degeneration. Retina. 2000; 20:244–50.
4. Jonas JB, Hayler JK, Panda-Jonas S. Intravitreal injection of crystalline cortisone as adjunctive treatment of proliferative vitreoretinopathy. Br J Ophthalmol. 2000; 84:1064–7.

5. Beer PM, Bakri SJ, Singh RJ, et al. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single injection. Ophthalmology. 2003; 110:681–6.
6. Jonas JB. Intraocular availability of triamcinolone acetonide after intravitreal injection. Am J Ophthalmol. 2004; 137:560–2.

7. Jonas JB, Kreissig I, Degenring R. Secondary chronic openangle glaucoma after intravitreal triamcinolone acetonide. Arch Ophthalmol. 2003; 121:729–30.

8. Lee EW, Hariprasad SM, Mieler WF, et al. Short term intraocular pressure trends after intravitreal triamcinolone injection. Am J Ophthalmol. 2007; 143:365–7.
9. Yang YH, Kim KR, Yang SW, Yim HB. The effect of intravitreal triamcinolone acetonide on intraocular pressure. J Korean Ophthalmol Soc. 2004; 45:1081–5.
10. Park HY, Yi KY, Kim HK. Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Korean J Ophthalmol. 2005; 19:122–7.

11. Kubota T, Okabe H, Hisatomi T, et al. Ultrastructure of the trabecular meshwork in secondary glaucoma eyes after intravitreal triamcinolone acetonide. J Glaucoma. 2006; 15:117–9.

12. Jonas JB, Kreissig I, Degenring R. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol. 2003; 87:24–7.

13. Machemer R. Five cases in which a depot steroid (hydrocortisone acetate and methylprednisolone acetate) was injected into the eye. Retina. 1996; 16:166–7.
14. Scholes GN, O'Brien WJ, Abrams GW, Kubicek MF. Clearance of triamcinolone from vitreous. Arch Ophthalmol. 1985; 103:1567–9.

15. Kim TH, Moon YS, Chin HS. Change of residual period and clearance rate of intravitreal triamcinolone according to initial dosage. J Korean Ophthalmol Soc. 2005; 46:1569–74.
16. Becker B, Bresnick G, Chevrette L, et al. Intraocular pressure and its response to topical corticosteroids in diabetes. Arch Ophthalmol. 1966; 76:477–83.

17. Becker B, Ballin N. Glaucoma and corticosteroid provocative testing. Arch Ophthalmol. 1965; 74:621–4.

18. Bigger JF, Palmberg PF, Zink H, Becker B. Sensitivity to glucocorticoids in primary open angle glaucoma. N Engl J Med. 1972; 287:992.
19. Dwinger MC, Pieper-Bodeewes I, Eter N, Holz FG. Variations in intraocular pressure (IOP) and necessity for paracentesis following intravitreal triamcinolone injection. Klin Monatsbl Augenheilkd. 2005; 222:638–42.
20. Seong HK, Lee JM, Park YS, Lee BR. Immediate natural course of IOP after IVTA and the effect of preoperative ocular massage. J Korean Ophthalmol Soc. 2007; 48:808–14.
21. Smithen LM, Ober MD, Maranan L, Spaide RF. Intravitreal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol. 2004; 138:740–3.

22. Barnebey HS, Robin AL, Zimmerman TJ, et al. The efficacy of brimonidine in decreasing elevations in intraocular pressure after laser trabeculoplasty. Ophthalmology. 1993; 100:1083–8.

23. Seong GJ, Lee YG, Lee JH, et al. Effect of 0.2% brimonidine in preventing intraocular pressure elevation after Nd:YAG laser posterior capsulotomy. Ophthal Surg Laser. 2000; 31:308–14.

24. Katz LJ. Twelve month evaluation of brimonidine purite versus brimonidine in patients with glaucoma or ocular hypertension. J Glaucoma. 2002; 11:119–26.
25. Wheeler LA, Gil DW, WoldeMussie E. Role of alpha‐2 adrenergic receptors in neuroprotection and glaucoma. Surv Ophthalmol. 2001; 45:S290–4.

26. Lai RK, Chun T, Hasson D, et al. Alpha‐2 adrenoceptor agonist protects retinal function after acute retinal ischemic injury in the rat. Vis Neurosci. 2002; 19:175–85.

27. Wheeler L, WoldeMussie E, Lai R. Role of alpha-2 agonists in neuroprotection. Surv Ophthalmol. 2003; 48:S47–51.

28. Benz MS, Albini TA, Holz ER, et al. Short term course of intraocular pressure after intravitreal injection of triamcinolone acetonide. Ophthalmology. 2006; 113:1174–8.
29. Lee EW, Hariprasad SM, Mieler WF, et al. Short term intraocular pressure trends after intravitreal triamcinolone injection. Am J Ophthalmol. 2007; 143:365–7.
30. Kotliar K, Maier M, Bauer S, et al. Effect of intravitreal injections and volume changes on intraocular pressure: clinical results and biomechanical model. Acta Ophthalmol Scand. 2007; 85:777–81.

31. Shields MB. Textbook of glaucoma. 4th ed.1. Baltimore: Williams & Wilkins;1998. p. 323.
Go to : 
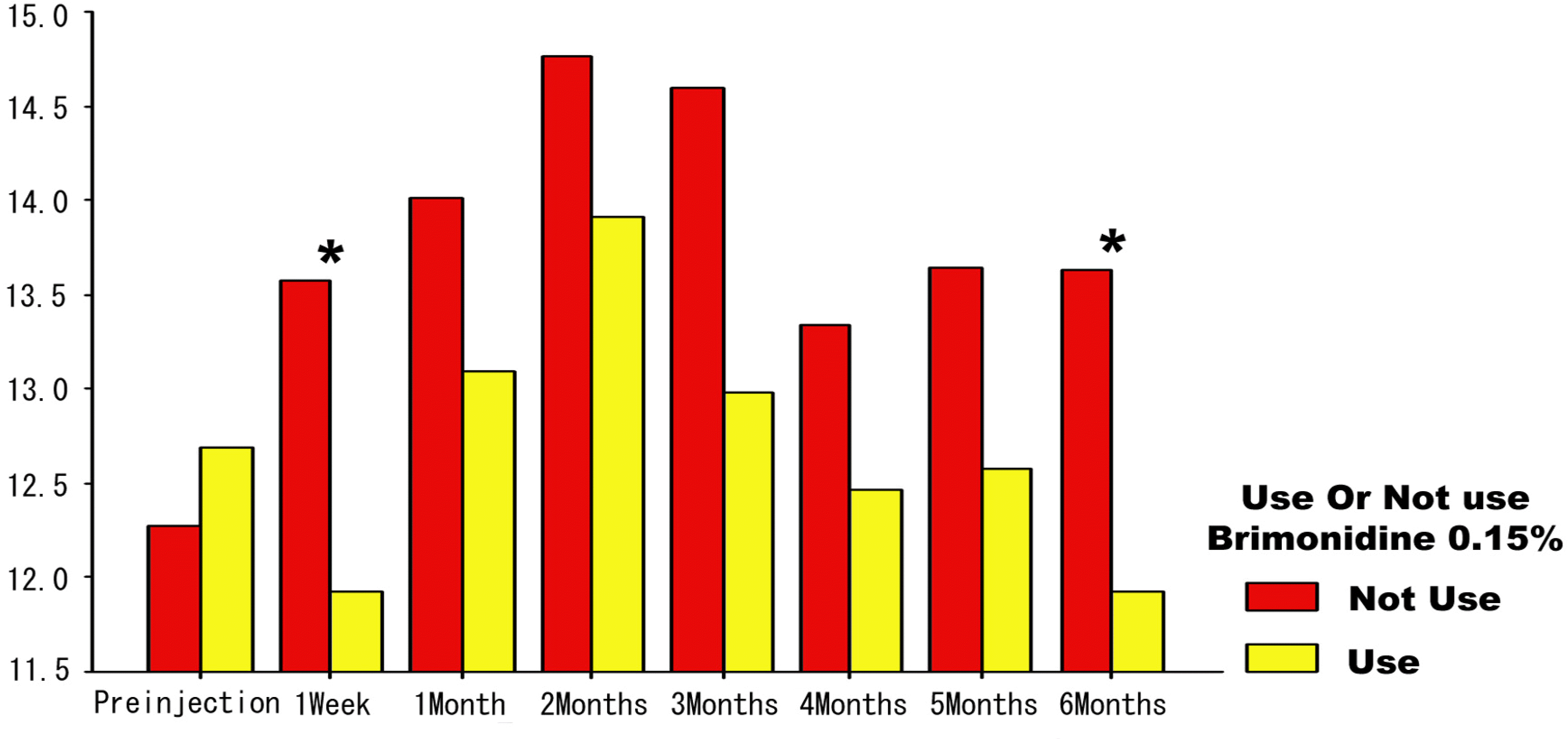 | Figure 1.Serial change of mean intraocular pressure in each group. ∗ Statistically significantly different between the two groups (p<0.05, Student's t-test). |
Table 1.
Patient demographics in each group
| Characteristics | Brimonidine 0.15% used | Brimonidine 0.15% not used | p-value |
|---|---|---|---|
| Number of eyes | 40 | 27 | |
| Age (years) | 59.7±13.2 | 57.2±12.2 | 0.435∗ |
|
Gender (male:female) |
25:15 |
13:14 |
0.317† |
|
Cause of macular edema |
|||
| NPDR | 13 | 10 | 0.795† |
| PDR | 8 | 3 | 0.504† |
| BRVO/CRVO | 14 | 8 | 0.792† |
Table 2.
Mean intraocular pressure (mmHg) at preinjection and 1 week, monthly until 6 months after intravitreal triamcinolone acetonide injection in each group
| Followup time |
Number of eyes |
IOP (mmHg) |
p-value∗ | ||||
|---|---|---|---|---|---|---|---|
| Use Brimonidine 0.15% | Not use Brimonidine 0.15% | Use Brimonidine 0.15% | p-value† | Not use Brimonidine 0.15% | p-value† | ||
| Pre-injection | 40 | 27 | 12.69±2.37 | 12.27±2.17 | 0.464 | ||
| 1 week | 40 | 27 | 11.93±3.36 | 0.036 | 13.58±3.25 | 0.006 | 0.049 |
| 1 Month | 40 | 27 | 13.10±3.23 | 0.285 | 14.02±2.52 | 0.000 | 0.219 |
| 2 Months | 40 | 27 | 13.92±3.78 | 0.008 | 14.77±4.32 | 0.002 | 0.396 |
| 3 Months | 36 | 24 | 12.98±4.63 | 0.372 | 14.59±4.67 | 0.009 | 0.193 |
| 4 Months | 34 | 21 | 12.46±3.89 | 0.825 | 13.35±3.11 | 0.030 | 0.382 |
| 5 Months | 33 | 21 | 12.57±4.43 | 0.747 | 13.65±3.74 | 0.025 | 0.358 |
| 6 Months | 32 | 20 | 11.93±2.37 | 0.193 | 13.64±2.94 | 0.006 | 0.026 |
Table 3.
Effects of DM, HTN, age, preinjection intraocular pressure on mean intraocular pressure after intravitreal injection of triamcinolone acetonide
|
IOP (mmHg) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-injection | 1 week | 1 month | 2 months | 3 months | 4 months | 5 months | 6 months | ||
| DM | + | 12.48±2.26 | 12.19±3.15 | 13.20±2.97 | 14.08±3.75 | 13.82±4.76 | 12.87±3.71 | 12.93±4.48 | 12.35±2.58 |
| (No.) | (38) | (38) | (38) | (38) | (35) | (33) | (32) | (31) | |
| - | 12.58±2.35 | 13.12±3.67 | 13.81±3.00 | 14.49±4.36 | 13.35±4.63 | 12.70±3.54 | 13.07±3.79 | 12.92±2.93 | |
| (No.) | (29) | (29) | (29) | (29) | (25) | (22) | (22) | (21) | |
| p-value∗ | 0.858 | 0.266 | 0.409 | 0.682 | 0.706 | 0.869 | 0.906 | 0.461 | |
| HTN | + | 12.42±2.36 | 12.46±3.65 | 13.55±3.04 | 14.39±3.42 | 13.09±4.39 | 12.89±4.25 | 12.64±3.49 | 12.22±2.56 |
| (No.) | (28) | (28) | (28) | (28) | (24) | (23) | (22) | (21) | |
| - | 12.60±2.25 | 12.68±3.24 | 13.41±2.96 | 14.17±4.40 | 13.98±4.87 | 12.73±3.14 | 13.23±4.62 | 12.83±2.82 | |
| (No.) | (39) | (39) | (39) | (39) | (36) | (32) | (32) | (31) | |
| p-value∗ | 0.755 | 0.795 | 0.842 | 0.826 | 0.472 | 0.870 | 0.614 | 0.438 | |
| Age | ≤60 | 13.11±2.29 | 13.05±3.52 | 14.21±3.39 | 15.30±4.68 | 14.57±5.03 | 12.97±3.22 | 12.82±3.36 | 12.74±2.62 |
| (No.) | (35) | (35) | (35) | (35) | (29) | (25) | (25) | (24) | |
| >60 | 11.87±2.11 | 12.09±3.22 | 12.66±2.23 | 13.12±2.72 | 12.74±4.20 | 12.66±3.95 | 13.14±4.82 | 12.45±2.83 | |
| (No.) | (32) | (32) | (32) | (32) | (31) | (30) | (29) | (28) | |
| p-value∗ | 0.026 | 0.253 | 0.030 | 0.022 | 0.133 | 0.750 | 0.778 | 0.700 | |
| IOP | ≥15 | 15.88±1.74 | 16.07±3.80 | 17.20±2.29 | 18.94±2.74 | 18.77±3.98 | 16.00±1.05 | 18.58±6.85 | 15.48±2.23 |
| (No.) | (10) | (10) | (10) | (10) | (6) | (5) | (5) | (5) | |
| <15 | 11.76±1.84 | 11.98±2.95 | 12.81±2.58 | 13.44±3.61 | 13.05±4.41 | 12.48±3.62 | 12.42±3.43 | 12.27±2.59 | |
| (No.) | (57) | (57) | (57) | (57) | (54) | (50) | (49) | (47) | |
| p-value∗ | 0.000 | 0.000 | 0.000 | 0.000 | 0.004 | 0.036 | 0.001 | 0.011 | |
Table 4.
Effects of Brimonidine 0.15% on intraocular pressure after intravitreal injection of triamcinolone acetonide in each DM, HTN group
Table 5.
Effects of brimonidine 0.15% on intraocular pressure after intravitreal injection of triamcinolone acetonide in each group
Table 6.
Comparison of mean intraocular pressure between preinjection and postinjections in each group
|
IOP (mmHg) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Brimonidine | Pre-injection | 1 week | 1 month | 2 months | 3 months | 4 months | 5 months | 6 months | |
| IOP | Use | 16.09±1.10 | 15.59±3.83 | 17.50±2.49 | 19.00±6.08 | 19.46±4.03 | 16.00±1.20 | 19.20±7.75 | 15.42±2.57 |
| ≥15 | p-value∗ | 0.711 | 0.215 | 0.011 | 0.156 | 0.873 | 0.448 | 0.577 | |
| Not Use | 16.50±0.92 | 18.00±4.24 | 16.00±0.42 | 18.92±2.09 | 15.3 | 16.0 | 16.1 | 15.7 | |
| p-value∗ | 0.638 | 0.389 | 0.618 | ||||||
| IOP | Use | 11.84±1.75 | 11.01±2.56 | 11.99±2.33 | 12.66±2.98 | 11.93±3.83 | 11.99±3.89 | 11.65±2.98 | 11.42±1.91 |
| <15 | p-value∗ | 0.012 | 0.690 | 0.099 | 0.828 | 0.812 | 0.640 | 0.262 | |
| Not Use | 11.93±1.85 | 13.22±2.99 | 13.86±2.55 | 14.43±4.13 | 14.56±4.77 | 13.21±3.13 | 13.53±3.79 | 13.52±2.98 | |
| p-value∗ | 0.009 | 0.000 | 0.003 | 0.009 | 0.031 | 0.026 | 0.005 | ||
Table 7.
The number of eyes that elevated intraocular pressure elevation more than 22 mmHg after intravitreal triamcinolone acetonide injection during the followup period in each group
| Followup time |
Number of eyes (%) |
p-value∗ | |
|---|---|---|---|
| Brimonidine 0.15% used | Brimonidine 0.15% not used | ||
| 3 Months | 4 (10) | 3 (11) | 0.594 |
| 4 Months | 2 (5) | 3 (11) | 0.317 |
| 5 Months | 1 (2.5) | 0 (0) | 0.597 |
| 6 Months | 1 (2.5) | 1 (4) | 0.647 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download