Abstract
Purpose
To assess whether a 4 mg/0.05 ml intravitreal triamcinolone acetonide injection can reduce the IOP elevation compared to conventional 4 mg/0.1 ml injection.
Methods
A retrospective case study was performed in 48 patients (48 eyes) who received intravitreal triamcinolone acetonide injection and who had a minimum follow-up time of six months. Patients were randomly assigned to receive 4 mg/0.1 ml or 4 mg/0.05 ml (24 patients in each group).
Results
Before injection, mean IOP was 13.8±2.2 mmHg and 13.9±2.4 mmHg in the 0.1 ml and 0.05 ml group. The difference in IOP elevation between the two groups was statistically significant immediately after injection (P=0.000), one hour after injection (P=0.001), and one day after injection (P=0.000). After injection, the central macular thickness decreased significantly the of two groups. The difference of the central macular thickness decrease between both groups was not statistically significant.
References
1. Machemer R, Sugita G, Tano Y. Treatment of intraocular proliferations with intravitreal steroids. Trans Am Ophthalmol Soc. 1979; 77:171–80.
2. Danis RP, Ciulla TA, Pratt LM, Anliker W. Intravitreal triamcinolone acetonide in exudative age‐ related macular degeneration. Retina. 2000; 20:244–50.
3. Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003; 136:791–6.

4. Wingate RJ, Beaumont PE. Intravitreal triamcinolone and elevated intraocular pressure. Aust N Z J Ophthalmol. 1999; 27:431–2.
5. Jonas JB, Kreissig I, Degenring R. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol. 2003; 87:24–7.

6. Özkiris A, Erkilic K. Complications of intravitreal injection of triamcinolone acetonide. Can J Ophthalmol. 2005; 40:63–8.
7. Yang YH, Kim KR, Yang SW, Yim HB. The effect of intravitreal triamcinolone acetonide on intraocular pressure. J Korean Ophthalmol Soc. 2004; 45:1081–5.
8. Smithen LM, Ober MD, Maranan L, Spaide RF. Intravitreal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol. 2004; 138:740–3.

9. Bakri SJ, Beer PM. The effect of intravitreal triamcinolone acetonide on intraocular pressure. Ophthalmic Surg Lasers Imaging. 2003; 34:386–90.

10. Park HY, Yi K, Kim HK. Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Korean J Ophthalmol. 2005; 19:122–7.

11. Jonas JB, Kreissig I, Degenring R. Secondary chronic open angle glaucoma after intravitreal triamcinolone acetonide. Arch Ophthalmol. 2003; 121:729–30.
12. Gilles MC, Simpson JM, Billson FA, et al. Safety of an intravitreal injection of triamcinolone:results from a randomized clinical trial. Arch Ophthalmol. 2004; 122:336–40.
13. Audren F, Lecleire‐ Collet A, Erginary A, et al. Intravitreal triamcinolone acetonide for diffuse diabetic macular edema: phase 2 trial comparing 4 mg vs 2 mg. Am J Ophthalmol. 2006; 142:794–9.

Figure 1.
(A) Sedimentation of triamcinolone acetonide. (B) Only 0.05 ml from the inferior layer suspension.
Figure 2.
Evolution of intraocular pressure after intravitreal triamcinolone acetonide 0.05 ml or 0.1 ml injection.
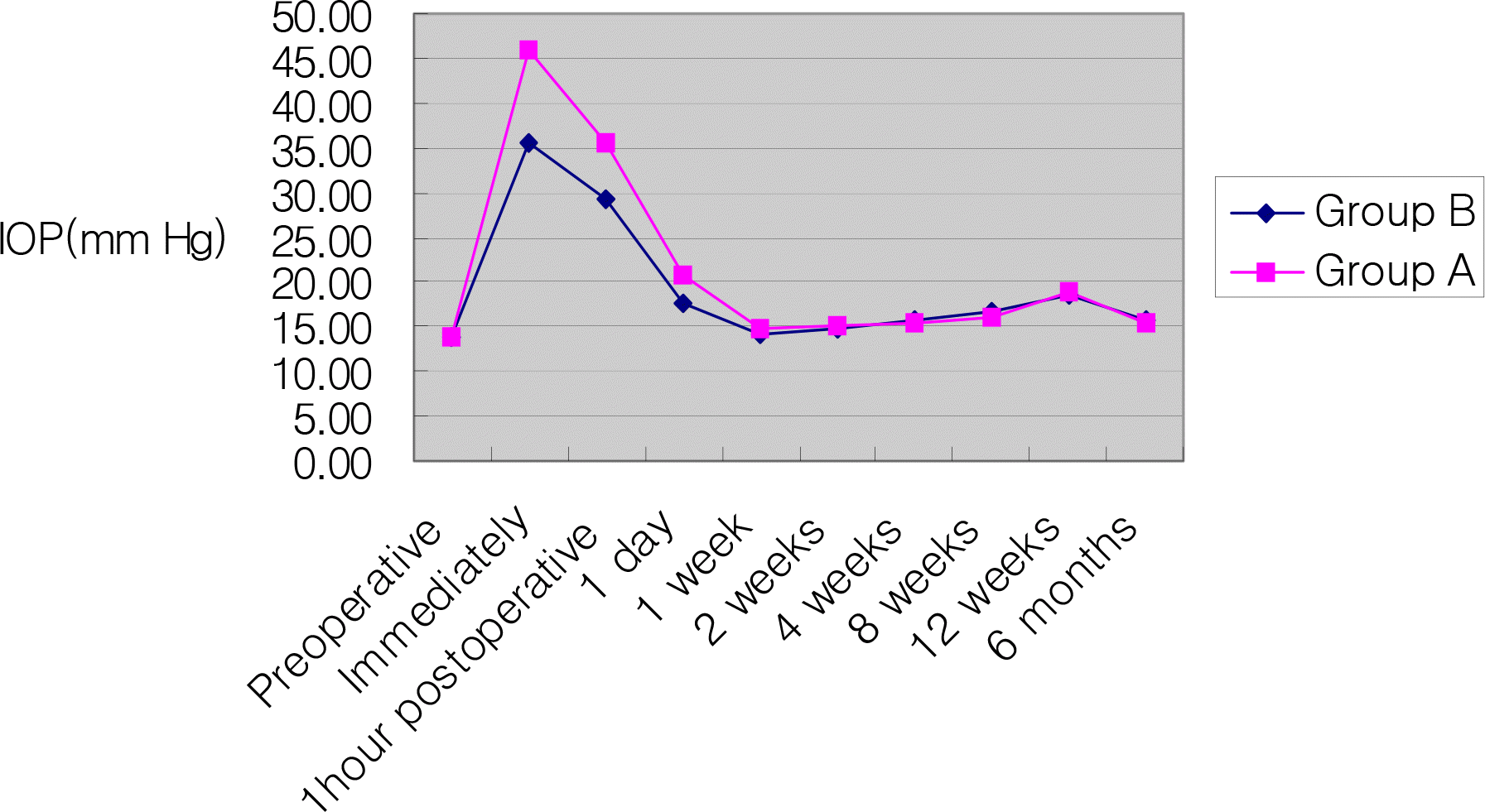
Figure 3.
Serial change in mean central macular thickness in eyes of 0.1 ml and 0.05 ml triamcinolone acetonide injection.
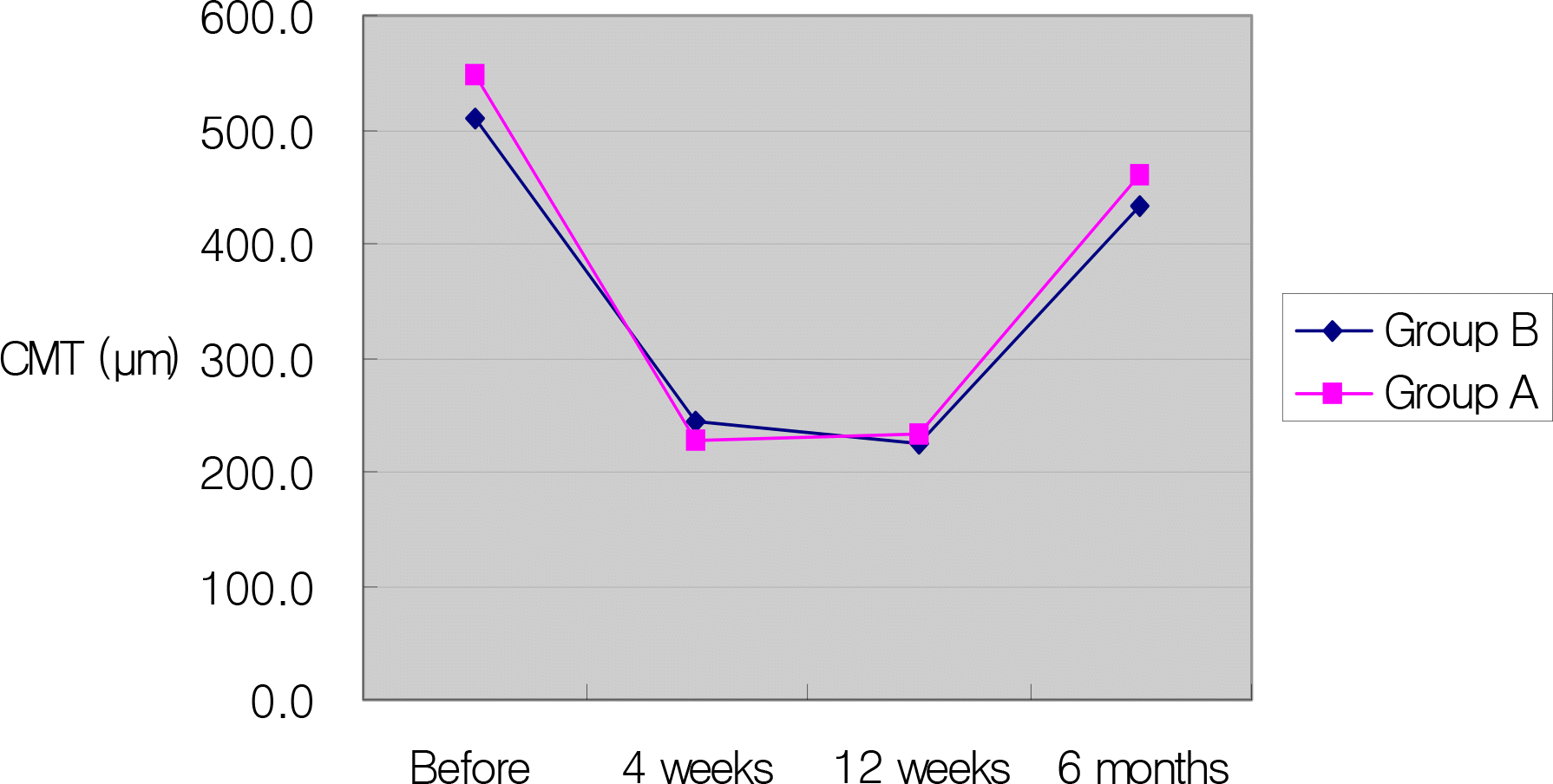
Table 1.
Clinical characteristics of 48 patients receiving intravitreal injection of triamcinolone acetonide
| Charateristics | Group A* | Grou B† | P-value |
|---|---|---|---|
| Number of eyes | 24 | 24 | |
| Age (years) | 59.7±7.9 | 57.1±10.1 | 0.25‡ |
| Gender (male:female) | 10:14 | 11:13 | 0.77§ |
| Lens (phakia:pseudophakia) | 17:7 | 18:6 | 0.75§ |
| Underlying disease | | | 0.86§ |
| Diabetic retinopathy | 17 | 17 | |
| CRVO∏ | 5 | 4 | |
| BRVO# | 2 | 3 | |
Table 2.
Mean (±SD*) intraocular pressure in eyes of 0.1 ml and 0.05 ml triamcinolone acetonide injection
| IOP†(mmHg) | Eyes injected with 0.1 ml (n=24) | Eyes injected with 0.05 ml (n=24) | P-value‡ of IOP difference between groups |
|---|---|---|---|
| Before injection | 13.8±2.2 | 13.9±2.4 | 0.721 |
| Immediately after injection | 45.8±4.8 | 35.7±5.2 | 0.000 |
| 1 hour | 35.5±3.2 | 29.2±3.4 | 0.001 |
| 1 day | 20.7±2.2 | 17.6±3.0 | 0.000 |
| 1 week | 14.7±1.4 | 14.2±2.7 | 0.195 |
| 2 weeks | 15.2±0.9 | 14.8±2.3 | 0.226 |
| 4 weeks | 15.5±1.3 | 15.6±2.2 | 0.983 |
| 8 weeks | 16.1±1.3 | 16.5±2.5 | 0.237 |
| 12 weeks | 18.9±1.3 | 18.5±1.8 | 0.736 |
| 6 months | 15.4±1.6 | 15.9±2.0 | 0.346 |
Table 3.
Mean (±SD*) central macular thickness in eyes of 0.1 ml and 0.05 ml triamcinolone acetonide injection
| CMT†(µm) | Eyes injected with 0.1 ml (n=24) | Eyes injected with 0.05 ml (n=24) | P-value‡ of CMT difference between groups |
|---|---|---|---|
| Before injection | 548.0±76.9 | 510.8±84.3 | 0.205 |
| 4 weeks | 228.3±29.0 | 243.8±37.7 | 0.093 |
| 12 weeks | 234.1±30.6 | 225.8±36.1 | 0.293 |
| 6 months | 459.1±49.9 | 432.3±62.6 | 0.069 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download